Abstract
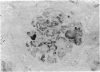
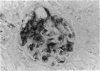
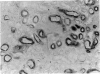
The distribution of proteoglycans in kidneys from patients with secondary (AA) systemic amyloidosis was investigated. Antisera reacting with the protein cores of chondroitin sulphate proteoglycan (CSPG), dermatan sulphate proteoglycan (DSPG) and heparan sulphate proteoglycan (HSPG) were used in conjunction with the peroxidase-antiperoxidase (PAP) method. HSPG was the only proteoglycan found to be specifically localized to the amyloid deposits. The staining was most intense on the endothelial side of the deposits in both the glomeruli and in the vessel walls. No staining was observed after absorption of the HSPG antiserum with a fraction of the amyloid preparations, corresponding in size to that reported for glomerular HSPG. The possible role of HSPG and endothelial cells in the pathogenesis of the amyloid deposits is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Eriksen N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4025–4028. doi: 10.1073/pnas.74.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson G., Dalferes E. R., Jr, Ruiz H., Radhakrishnamurthy B. Changes of mucopolysaccharides in the heart involved by amyloidosis. Am J Cardiol. 1969 Sep;24(3):358–364. doi: 10.1016/0002-9149(69)90429-9. [DOI] [PubMed] [Google Scholar]
- Binette P., Matsuzaki M., Calkins E., Alper R., Winzler R. J. Carbohydrate composition of amyloid components. Proc Soc Exp Biol Med. 1971 May;137(1):165–167. doi: 10.3181/00379727-137-35536. [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Skinner M., Cohen A. S. Characterization of the mucopolysaccharides associated with fractions of guanidine-denatured amyloid fibrils. Clin Chim Acta. 1974 Sep 30;55(3):295–305. doi: 10.1016/0009-8981(74)90003-5. [DOI] [PubMed] [Google Scholar]
- Cohen A. S., Shirahama T. Electron microscopic analysis of isolated amyloid fibrils from patients with primary, secondary and myeloma-associated disease. A study utilizing shadowing and negative staining techniques. Isr J Med Sci. 1973 Jul;9(7):849–856. [PubMed] [Google Scholar]
- Eanes E. D., Glenner G. G. X-ray diffraction studies on amyloid filaments. J Histochem Cytochem. 1968 Nov;16(11):673–677. doi: 10.1177/16.11.673. [DOI] [PubMed] [Google Scholar]
- Ein D., Kimura S., Terry W. D., Magnotta J., Glenner G. G. Amino acid sequence of an amyloid fibril protein of unknown origin. J Biol Chem. 1972 Sep 10;247(17):5653–5655. [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G., Eanes E. D., Bladen H. A., Linke R. P., Termine J. D. Beta-pleated sheet fibrils. A comparison of native amyloid with synthetic protein fibrils. J Histochem Cytochem. 1974 Dec;22(12):1141–1158. doi: 10.1177/22.12.1141. [DOI] [PubMed] [Google Scholar]
- Glenner G. G., Ein D., Eanes E. D., Bladen H. A., Terry W., Page D. L. Creation of "amyloid" fibrils from Bence Jones proteins in vitro. Science. 1971 Nov 12;174(4010):712–714. doi: 10.1126/science.174.4010.712. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Hascall V. C., Farquhar M. G. Partial characterization of newly synthesized proteoglycans isolated from the glomerular basement membrane. J Cell Biol. 1981 Aug;90(2):527–532. doi: 10.1083/jcb.90.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Franklin E. C., Frangione B., Pras M. The amino acid sequence of a major nonimmunoglobulin component of some amyloid fibrils. J Clin Invest. 1972 Oct;51(10):2773–2776. doi: 10.1172/JCI107098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaki K., Murao S., Tsunetoshi S., Koizumi N., Isobe T., Nakamura N., Nakano H., Ogino T., Hosokawa S. Primary systemic amyloidosis. A case permitting pathological and biochemical investigations. Acta Pathol Jpn. 1979 Jan;29(1):157–169. doi: 10.1111/j.1440-1827.1979.tb01299.x. [DOI] [PubMed] [Google Scholar]
- Mowry R. W., Scott J. E. Observations on the basophilia of amyloids. Histochemie. 1967;10(1):8–32. doi: 10.1007/BF00304372. [DOI] [PubMed] [Google Scholar]
- Norling B., Glimelius B., Wasteson A. A chondroitin sulphate proteoglycan from human cultured glial and glioma cells. Structural and functional properties. Biochem J. 1984 Aug 1;221(3):845–853. doi: 10.1042/bj2210845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling B., Glimelius B., Wasteson A. Heparan sulfate proteoglycan of cultured cells: demonstration of a lipid- and a matrix-associated form. Biochem Biophys Res Commun. 1981 Dec 31;103(4):1265–1272. doi: 10.1016/0006-291x(81)90259-x. [DOI] [PubMed] [Google Scholar]
- Oohira A., Wight T. N., Bornstein P. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J Biol Chem. 1983 Feb 10;258(3):2014–2021. [PubMed] [Google Scholar]
- Parthasarathy N., Spiro R. G. Isolation and characterization of the heparan sulfate proteoglycan of the bovine glomerular basement membrane. J Biol Chem. 1984 Oct 25;259(20):12749–12755. [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C. Isolation of amyloid P component (protein AP) from normal serum as a calcium-dependent binding protein. Lancet. 1977 May 14;1(8020):1029–1031. doi: 10.1016/s0140-6736(77)91260-0. [DOI] [PubMed] [Google Scholar]
- Pollak A., Coradello H., Latzka U., Lischka A., Lubec G. Wechselwirkungen zwischen Amyloid P und Bindegewebsproteinen. Wien Klin Wochenschr. 1982 May 28;94(11):291–293. [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M., Reshef T. The acid-soluble fraction of amyloid--a fibril forming protein. Biochim Biophys Acta. 1972 Jun 22;271(1):193–203. doi: 10.1016/0005-2795(72)90147-x. [DOI] [PubMed] [Google Scholar]
- Pras M., Zucker-Franklin D., Rimon A., Franklin E. C. Physical, chemical, and ultrastructural studies of water-soluble human amyloid fibrils. Comparative analyses of nine amyloid preparations. J Exp Med. 1969 Oct 1;130(4):777–796. doi: 10.1084/jem.130.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M., Shirahama T., Cohen A. S., Deal C. L. The association of amyloid P-component (AP) with the amyloid fibril: an updated method for amyloid fibril protein isolation. Prep Biochem. 1982;12(5):461–476. doi: 10.1080/10826068208070597. [DOI] [PubMed] [Google Scholar]
- Sletten K., Husby G., Natvig J. B. The complete amino acid sequence of an amyloid fibril protein AA1 of unusual size (64 residues). Biochem Biophys Res Commun. 1976 Mar 8;69(1):19–25. doi: 10.1016/s0006-291x(76)80266-5. [DOI] [PubMed] [Google Scholar]
- Sletten K., Husby G. The complete amino-acid sequence of non-immunoglobulin amyloid fibril protein AS in rheumatoid arthritis. Eur J Biochem. 1974 Jan 3;41(1):117–125. doi: 10.1111/j.1432-1033.1974.tb03251.x. [DOI] [PubMed] [Google Scholar]
- Snow A. D., Kisilevsky R. Temporal relationship between glycosaminoglycan accumulation and amyloid deposition during experimental amyloidosis. A histochemical study. Lab Invest. 1985 Jul;53(1):37–44. [PubMed] [Google Scholar]
- Stiller D., Katenkamp D. Demonstration of orderly arranged acidic groups in amyloid by alcian blue. Histochemistry. 1974 Apr 22;39(2):163–169. doi: 10.1007/BF00492045. [DOI] [PubMed] [Google Scholar]
- Stow J. L., Glasgow E. F., Handley C. J., Hascall V. C. Biosynthesis of proteoglycans by isolated rabbit glomeruli. Arch Biochem Biophys. 1983 Sep;225(2):950–957. doi: 10.1016/0003-9861(83)90110-8. [DOI] [PubMed] [Google Scholar]
- Westermark G. T., Westermark P., Sletten K. Amyloid fibril protein AA. Characterization of uncommon subspecies from a patient with rheumatoid arthritis. Lab Invest. 1987 Jul;57(1):57–64. [PubMed] [Google Scholar]
- Westermark P. The heterogeneity of protein AA in secondary (reactive)systemic amyloidosis. Biochim Biophys Acta. 1982 Feb 4;701(1):19–23. doi: 10.1016/0167-4838(82)90306-5. [DOI] [PubMed] [Google Scholar]