Abstract
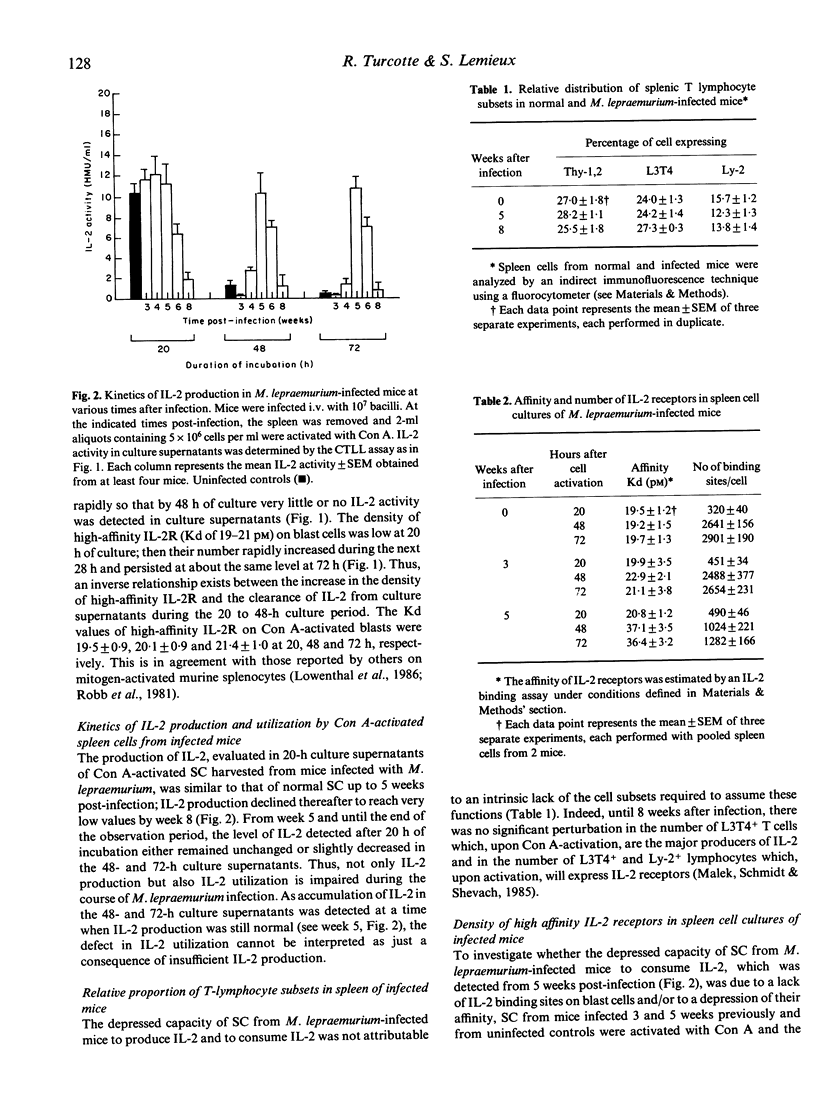
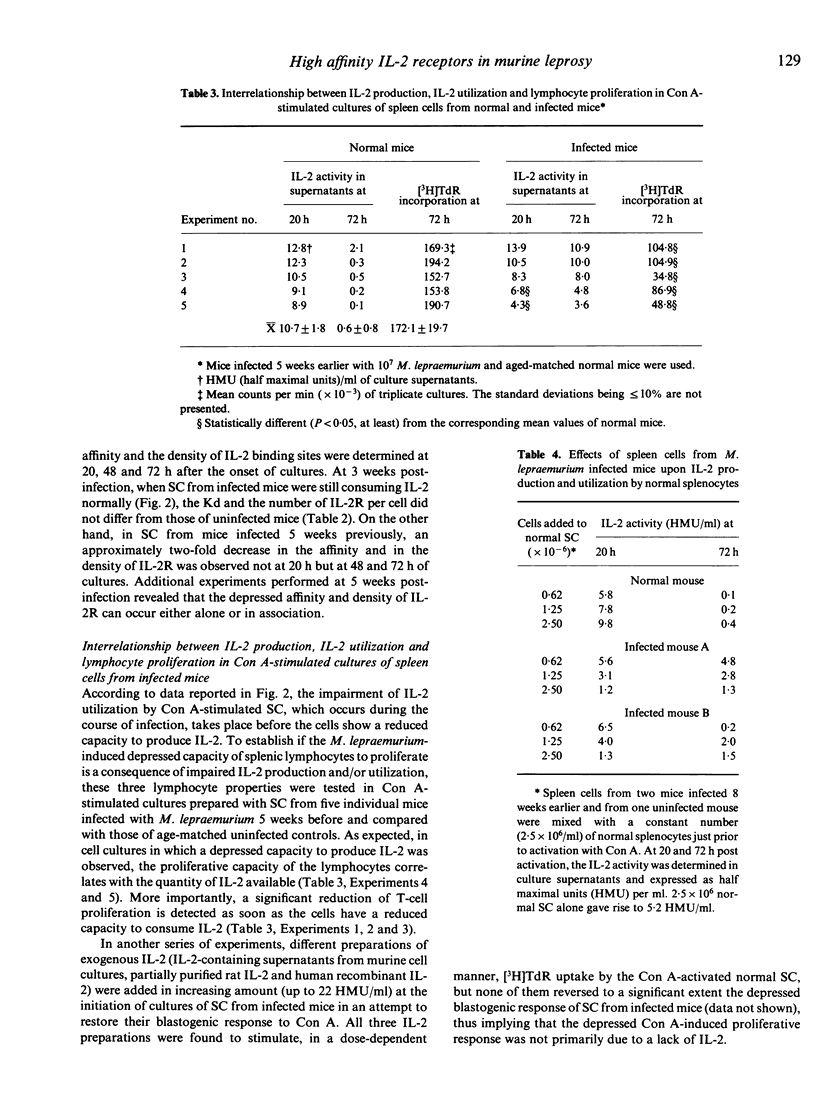
The production of interleukin-2 (IL-2) by Con A-activated spleen cells (SC) progressively declined and reached negligible values during the course of infection of C57BL/6 mice with Mycobacterium lepraemurium. In addition, the capacity of cultured SC to utilize IL-2 was highly reduced, as demonstrated by the accumulation of IL-2 activity in culture supernatants at 48 and 72 h after Con A activation. The depressed IL-2 utilization started to be observed about 1 to 2 weeks prior to the onset of the depressed IL-2 production and was not reversed by the addition of exogenous IL-2; thus implying that a lack of IL-2 utilization rather than a lack of IL-2 production could be directly responsible for the inhibition of T-cell proliferative responses to Con A in SC cultures of infected mice. The utilization of IL-2 was found to be down-regulated, at least in part, by splenic suppressor cells since, in mixed-culture experiments, SC from infected mice actively depressed the capacity of normal splenocytes to consume IL-2. Finally, the depressed IL-2 utilization would result from a 2- to 3-fold reduction of either or both the density of high-affinity IL-2 receptors and their affinity for IL-2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brett S. J. T-cell responsiveness in Mycobacterium lepraemurium infections in a "resistant" (CBA) and a "susceptible" (BALB/c) mouse strain. Cell Immunol. 1984 Nov;89(1):132–143. doi: 10.1016/0008-8749(84)90204-1. [DOI] [PubMed] [Google Scholar]
- Cantrell D. A., Smith K. A. The interleukin-2 T-cell system: a new cell growth model. Science. 1984 Jun 22;224(4655):1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- Carlsson R., Sjögren H. O. Kinetics of IL-2 and interferon-gamma production, expression of IL-2 receptors, and cell proliferation in human mononuclear cells exposed to staphylococcal enterotoxin A. Cell Immunol. 1985 Nov;96(1):175–183. doi: 10.1016/0008-8749(85)90349-1. [DOI] [PubMed] [Google Scholar]
- Colizzi V., Ferluga J., Garreau F., Malkovsky M., Asherson G. L. Suppressor cells induced by BCG release non-specific factors in vitro which inhibit DNA synthesis and interleukin-2 production. Immunology. 1984 Jan;51(1):65–71. [PMC free article] [PubMed] [Google Scholar]
- Deschenes M., Guenounou M., Ronco E., Vacheron F., Nauciel C. Impairment of lymphocyte proliferative responses and interleukin-2 production in susceptible (C57BL/6) mice infected with Salmonella typhimurium. Immunology. 1986 Jun;58(2):225–230. [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Ha D. K., Lawton J. W., Gardner I. D. Immunosuppressive activities of peritoneal and splenic macrophages in murine leprosy: effect on lymphocyte transformation and tumor growth. Microbiol Immunol. 1984;28(7):793–806. doi: 10.1111/j.1348-0421.1984.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Harel-Bellan A., Joskowicz M., Fradelizi D., Eisen H. Modification of T-cell proliferation and interleukin 2 production in mice infected with Trypanosoma cruzi. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3466–3469. doi: 10.1073/pnas.80.11.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Deficit of interleukin 2 production associated with impaired T-cell proliferative responses in Mycobacterium lepraemurium infection. Infect Immun. 1983 Jan;39(1):109–116. doi: 10.1128/iai.39.1.109-116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Influence of dose and route of Mycobacterium lepraemurium inoculation on the production of interleukin 1 and interleukin 2 in C57BL/6 mice. Infect Immun. 1984 Jun;44(3):665–671. doi: 10.1128/iai.44.3.665-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Surface Lyt phenotype of suppressor cells in C57BL/6 mice infected with Mycobacterium lepraemurium. Clin Exp Immunol. 1983 Oct;54(1):151–157. [PMC free article] [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Larsson E. L. Mechanism of T cell activation. II. Antigen- and lectin-dependent acquisition of responsiveness to TCGF is a nonmitogenic, active response of resting T cells. J Immunol. 1981 Apr;126(4):1323–1326. [PubMed] [Google Scholar]
- Lowenthal J. W., Corthésy P., Tougne C., Lees R., MacDonald H. R., Nabholz M. High and low affinity IL 2 receptors: analysis by IL 2 dissociation rate and reactivity with monoclonal anti-receptor antibody PC61. J Immunol. 1985 Dec;135(6):3988–3994. [PubMed] [Google Scholar]
- Lowenthal J. W., Howe R. C., Ceredig R., MacDonald H. R. Functional status of interleukin 2 receptors expressed by immature (Lyt-2-/L3T4-) thymocytes. J Immunol. 1986 Oct 15;137(8):2579–2584. [PubMed] [Google Scholar]
- Malek T. R., Schmidt J. A., Shevach E. M. The murine IL 2 receptor. III. Cellular requirements for the induction of IL 2 receptor expression on T cell subpopulations. J Immunol. 1985 Apr;134(4):2405–2413. [PubMed] [Google Scholar]
- Mohagheghpour N., Gelber R. H., Larrick J. W., Sasaki D. T., Brennan P. J., Engleman E. G. Defective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with defective expression of interleukin 2 receptors and is not reconstituted by interleukin 2. J Immunol. 1985 Aug;135(2):1443–1449. [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Navalkar R. G., Patel P. J., Kanchana M. V. Studies on immune response to Mycobacterium lepraemurium. Evaluation of the cell-mediated immune response in mice. Int Arch Allergy Appl Immunol. 1980;62(4):423–432. [PubMed] [Google Scholar]
- Osawa H., Josimovic-Alasevic O., Diamantstein T. Interleukin 2 receptors are released by cells in vitro and in vivo. I. Detection of soluble IL 2 receptors in cell culture supernatants and in the serum of mice by an immunoradiometric assay. Eur J Immunol. 1986 Apr;16(4):467–469. doi: 10.1002/eji.1830160426. [DOI] [PubMed] [Google Scholar]
- Reiner N. E., Finke J. H. Interleukin 2 deficiency in murine Leishmaniasis donovani and its relationship to depressed spleen cell responses to phytohemagglutinin. J Immunol. 1983 Sep;131(3):1487–1491. [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Saito N., Hirooka Y. Evidence for generation of suppressor T cells in Mycobacterium lepraemurium-infected CBA/J mice, a mouse strain highly susceptible to the infection. Microbiol Immunol. 1983;27(1):75–85. doi: 10.1111/j.1348-0421.1983.tb03569.x. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Gillis S., Baker P. E., McKenzie D., Ruscetti F. W. T-cell growth factor-mediated T-cell proliferation. Ann N Y Acad Sci. 1979;332:423–432. doi: 10.1111/j.1749-6632.1979.tb47136.x. [DOI] [PubMed] [Google Scholar]
- Toossi Z., Kleinhenz M. E., Ellner J. J. Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med. 1986 May 1;163(5):1162–1172. doi: 10.1084/jem.163.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R., Legault D. Mechanisms underlying the depressed production of interleukin-2 in spleen and lymph node cell cultures of mice infected with Mycobacterium bovis BCG. Infect Immun. 1986 Mar;51(3):826–831. doi: 10.1128/iai.51.3.826-831.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte R. Suppressor cells in experimental murine leprosy. Int J Lepr Other Mycobact Dis. 1978 Jul-Dec;46(3-4):358–363. [PubMed] [Google Scholar]
- Yamaura N., Akiyama T., Nakano T. Mitogen-induced DNA synthesis in various mouse strains infected with a large or small dose of murine leprosy bacilli. Microbiol Immunol. 1981;25(3):245–255. doi: 10.1111/j.1348-0421.1981.tb00027.x. [DOI] [PubMed] [Google Scholar]


