Abstract
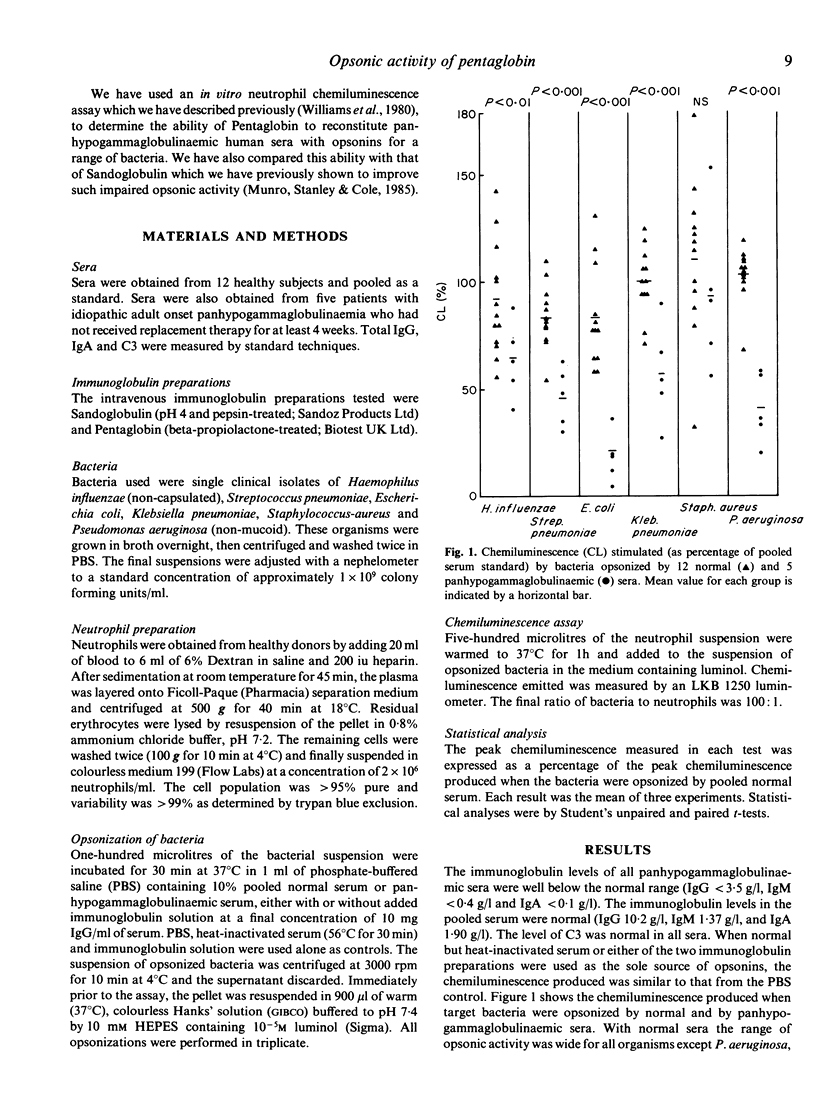
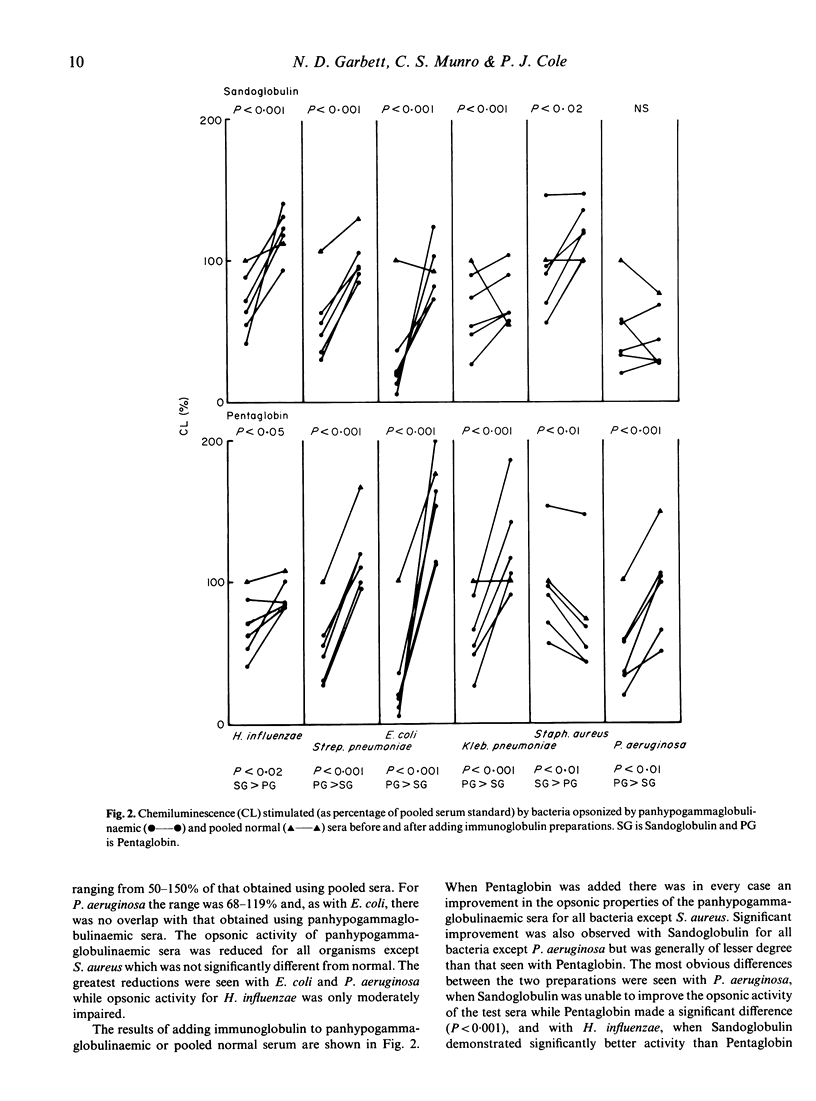
Standard preparations of immunoglobulin for intravenous use consist predominantly of IgG (greater than 95%). We have compared the ability of a standard preparation (Sandoglobulin) with that of a new preparation (Pentaglobin, containing 12% IgM and 12% IgA) to improve the opsonic activity of antibody-deficient human sera in vitro. Panhypogammaglobulinaemic sera were poorly opsonic for five of six organisms tested, particularly Pseudomonas aeruginosa, Escherichia coli and Streptococcus pneumoniae, but opsonized Staphylococcus aureus almost normally. Both immunoglobulin preparations significantly improved the opsonic activity of the antibody-deficient sera for most organisms. The major difference between the two preparations was the ability of Pentaglobin to supply opsonins for P. aeruginosa, E. coli and Klebsiella pneumoniae, while Sandogloblin was significantly more potent in opsonins for Haemophilus influenzae. Pentaglobin demonstrates significant in vitro opsonic activity, particularly for enterobacteria (coliforms) and P. aeruginosa. Its content of IgM antibodies appears to confer special properties on Pentaglobin not seen with standard preparations of immunoglobulin for intravenous use. Its place in clinical practice remains to be determined but it may have a possible role in augmenting host defence mechanisms in Gram-negative septicaemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Collins M. S., Roby R. E. Protective activity of an intravenous immune globulin (human) enriched in antibody against lipopolysaccharide antigens of Pseudomonas aeruginosa. Am J Med. 1984 Mar 30;76(3A):168–174. doi: 10.1016/0002-9343(84)90337-1. [DOI] [PubMed] [Google Scholar]
- Cross A., Allen J. R., Burke J., Ducel G., Harris A., John J., Johnson D., Lew M., MacMillan B., Meers P. Nosocomial infections due to Pseudomonas aeruginosa: review of recent trends. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S837–S845. doi: 10.1093/clinids/5.supplement_5.s837. [DOI] [PubMed] [Google Scholar]
- Jones R. J., Roe E. A., Gupta J. L. Controlled trials of a polyvalent pseudomonas vaccine in burns. Lancet. 1979 Nov 10;2(8150):977–982. doi: 10.1016/s0140-6736(79)92559-5. [DOI] [PubMed] [Google Scholar]
- McGowan J. E., Jr Changing etiology of nosocomial bacteremia and fungemia and other hospital-acquired infections. Rev Infect Dis. 1985 Jul-Aug;7 (Suppl 3):S357–S370. doi: 10.1093/clinids/7.supplement_3.s357. [DOI] [PubMed] [Google Scholar]
- Munro C. S., Stanley P. J., Cole P. J. Assessment of biological activity of immunoglobulin preparations by using opsonized micro-organisms to stimulate neutrophil chemiluminescence. Clin Exp Immunol. 1985 Jul;61(1):183–188. [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Small G. J. Passive immune therapy for experimental Pseudomonas aeruginosa pneumonia in the neutropenic host. J Infect Dis. 1987 May;155(5):973–978. doi: 10.1093/infdis/155.5.973. [DOI] [PubMed] [Google Scholar]
- Pollack M. Antibody activity against Pseudomonas aeruginosa in immune globulins prepared for intravenous use in humans. J Infect Dis. 1983 Jun;147(6):1090–1098. doi: 10.1093/infdis/147.6.1090. [DOI] [PubMed] [Google Scholar]
- Pollack M., Huang A. I., Prescott R. K., Young L. S., Hunter K. W., Cruess D. F., Tsai C. M. Enhanced survival in Pseudomonas aeruginosa septicemia associated with high levels of circulating antibody to Escherichia coli endotoxin core. J Clin Invest. 1983 Dec;72(6):1874–1881. doi: 10.1172/JCI111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Young L. S. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosa septicemia in man. J Clin Invest. 1979 Feb;63(2):276–286. doi: 10.1172/JCI109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer J., Späth P. J., Skvaril F., Nydegger U. E. Characterization of various immunoglobulin preparations for intravenous application. II. Complement activation and binding to staphylococcus protein A. Vox Sang. 1982 Feb;42(2):74–80. doi: 10.1159/000460851. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Stevens P., Young L. S. Quantitative granulocyte chemiluminescence in the rapid detection of impaired opsonization of Escherichia coli. Infect Immun. 1977 Jun;16(3):796–804. doi: 10.1128/iai.16.3.796-804.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehm E. R., Ashida E., Kim K. S., Winston D. J., Haas A., Gale R. P. Intravenous immunoglobulins as therapeutic agents. Ann Intern Med. 1987 Sep;107(3):367–382. doi: 10.7326/0003-4819-107-2-367. [DOI] [PubMed] [Google Scholar]
- Weinstein R. J., Young L. S. Neutrophil function in gram-negative rod bacteremia. The interaction between phagocytic cells, infecting organisms, and humoral factors. J Clin Invest. 1976 Jul;58(1):190–199. doi: 10.1172/JCI108449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. J., Hastings M. J., Easmon C. S., Cole P. J. Factor affecting the in vitro assessment of opsonization: a study of the kinetics of opsonization using the technique of phagocytic chemiluminescence. Immunology. 1980 Dec;41(4):903–911. [PMC free article] [PubMed] [Google Scholar]
- Yap P. L. The use of intravenous immunoglobulin for the treatment of infection: an overview. J Infect. 1987 Jul;15 (Suppl 1):21–28. doi: 10.1016/s0163-4453(87)92436-4. [DOI] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]
- Young L. S. Role of antibody in infections due to Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S111–S118. doi: 10.1093/infdis/130.supplement.s111. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- van Furth R., Leijh P. C., Klein F. Correlation between opsonic activity for various microorganisms and composition of gammaglobulin preparations for intravenous use. J Infect Dis. 1984 Apr;149(4):511–517. doi: 10.1093/infdis/149.4.511. [DOI] [PubMed] [Google Scholar]


