Abstract
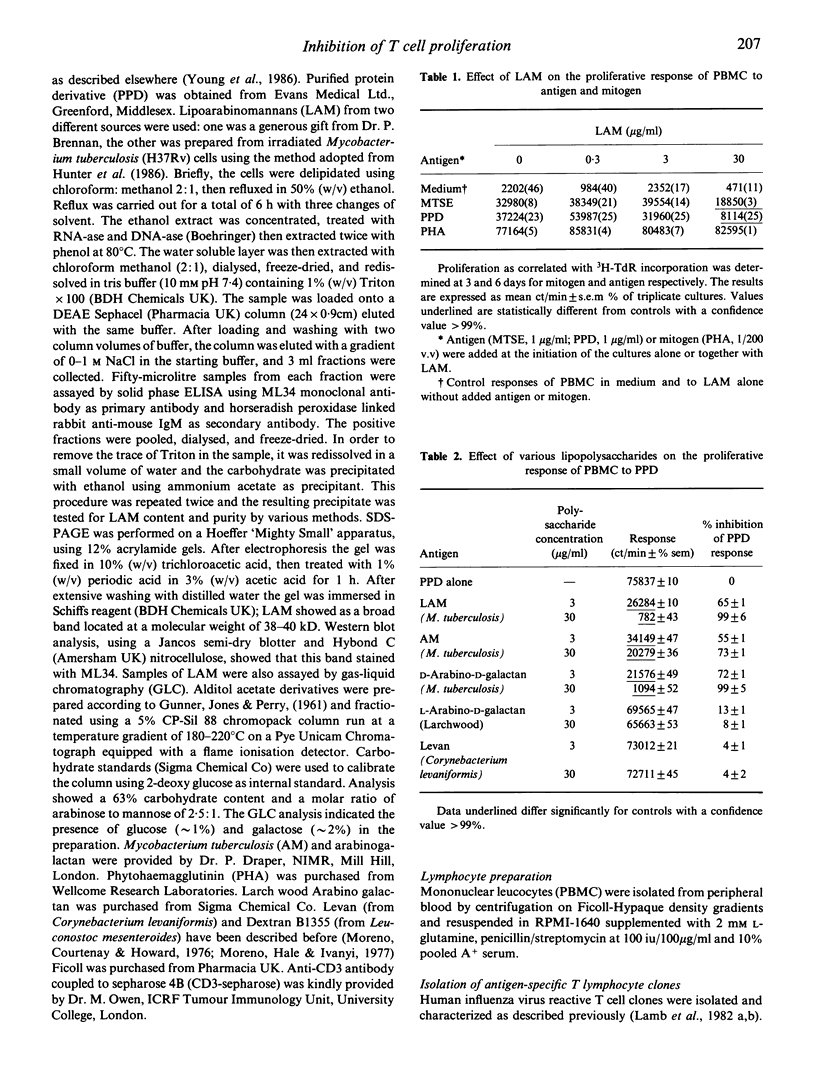
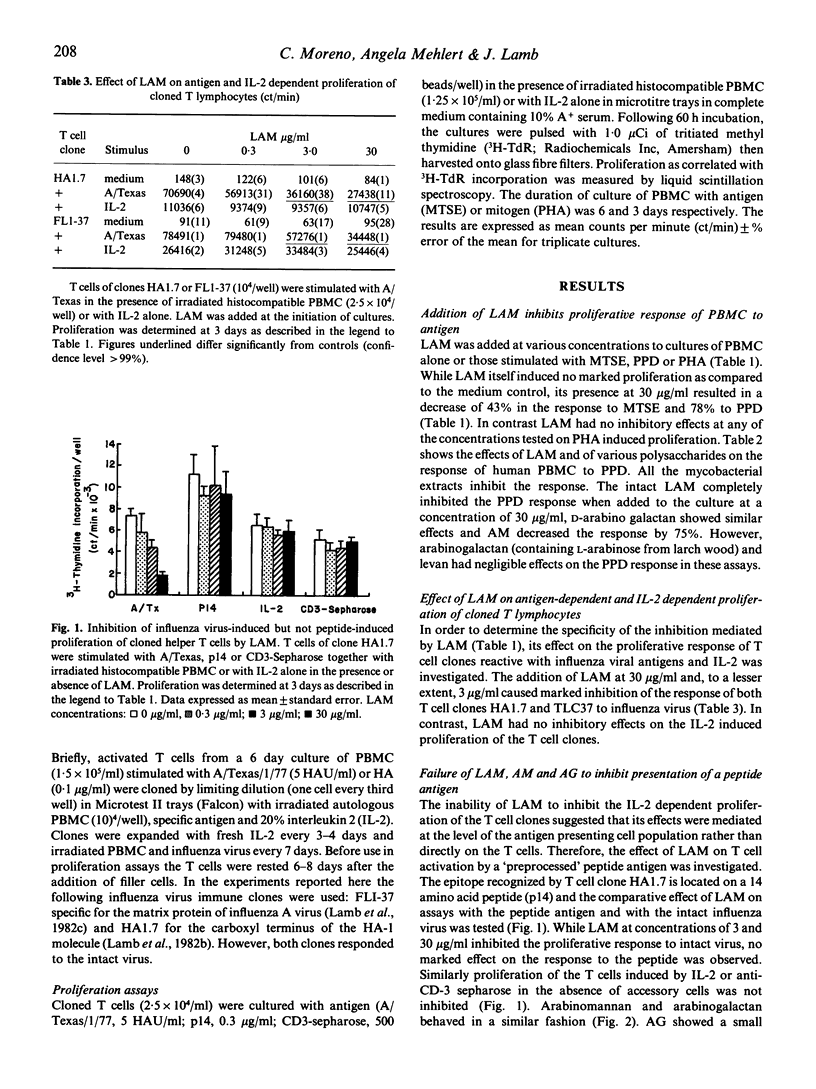
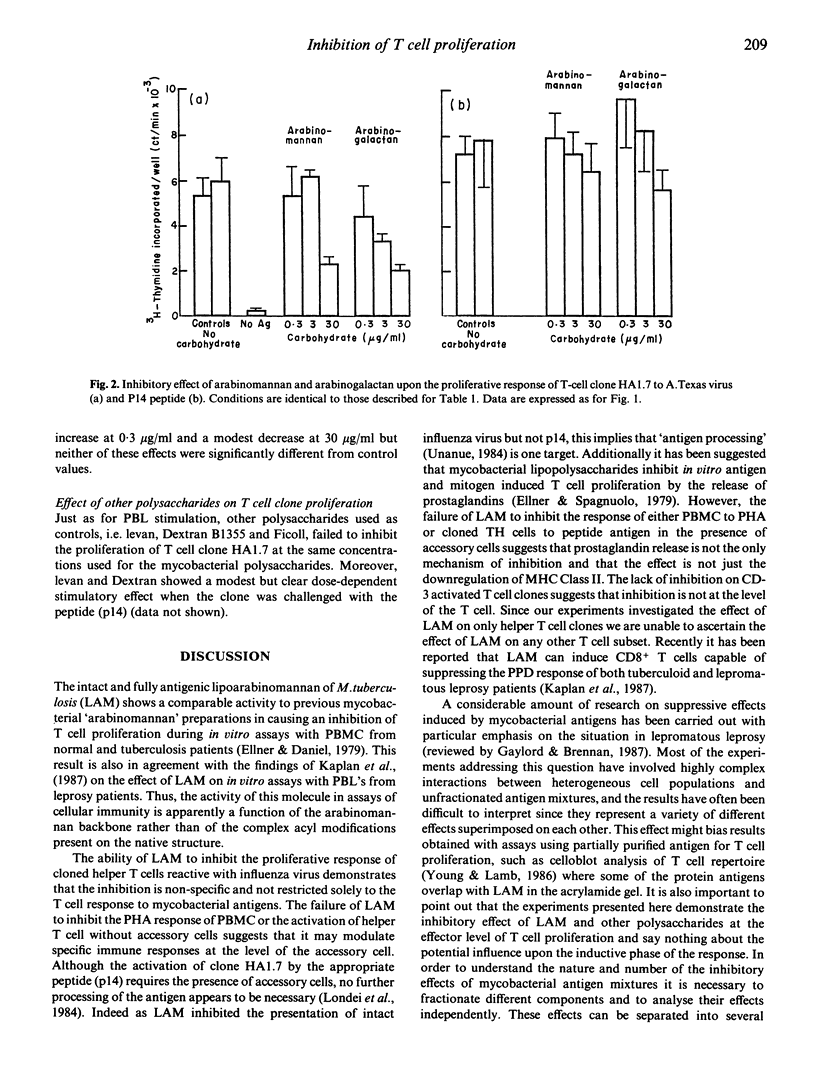
Lipoarabinomannan from Mycobacterium tuberculosis was able to inhibit antigen induced T cell proliferation of human CD4+ T cell clones specific for influenza virus. The inhibitory effect was also present when peripheral human T cells were stimulated with crude mycobacterial antigen extracts. Non-specific T cell stimulation, i.e. IL-2, PHA and anti-CD3 antibodies coupled to beads, was not affected. The inhibitory property was also found when arabinomannan and arabinogalactan of mycobacterial origin were tested but not with other unrelated polysaccharides used as controls. The effect appears to be related to the processing of the antigen by the antigen-presenting cells, since it was evident when T cell clones were stimulated with whole virus, whereas stimulation with a synthetic peptide containing the relevant epitope was not inhibitable.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Gaylord H., Brennan P. J., Young D. B., Buchanan T. M. Most Mycobacterium leprae carbohydrate-reactive monoclonal antibodies are directed to lipoarabinomannan. Infect Immun. 1987 Nov;55(11):2860–2863. doi: 10.1128/iai.55.11.2860-2863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. W., Gaylord H., Brennan P. J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986 Sep 15;261(26):12345–12351. [PubMed] [Google Scholar]
- Kaplan G., Gandhi R. R., Weinstein D. E., Levis W. R., Patarroyo M. E., Brennan P. J., Cohn Z. A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987 May 1;138(9):3028–3034. [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Johnson A. H., Hartzman R. J., Woody J. N. Antigen-specific human T lymphocyte clones: induction, antigen specificity, and MHC restriction of influenza virus-immune clones. J Immunol. 1982 Jan;128(1):233–238. [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Woody J. N., Green N. Human T-cell clones recognize chemically synthesized peptides of influenza haemagglutinin. Nature. 1982 Nov 4;300(5887):66–69. doi: 10.1038/300066a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Phelan M., Lake P., Woody J. N. Antigen-specific human T lymphocyte clones: viral antigen specificity of influenza virus-immune clones. J Immunol. 1982 Mar;128(3):1428–1432. [PubMed] [Google Scholar]
- Misaki A., Azuma I., Yamamura Y. Structural and immunochemical studies on D-arabino-D-mannans and D-mannans of Mycobacterium tuberculosis and other Mycobacterium species. J Biochem. 1977 Dec;82(6):1759–1770. doi: 10.1093/oxfordjournals.jbchem.a131874. [DOI] [PubMed] [Google Scholar]
- Moreno C., Courtenay B. M., Howard J. G. Molecular size and structure in relation to the tolerogenicity of small fructosans (levans). Immunochemistry. 1976 May;13(5):429–435. doi: 10.1016/0019-2791(76)90379-7. [DOI] [PubMed] [Google Scholar]
- Moreno C., Hale C., Ivanyi L. The mitogenic, immunogenic and tolerogenic properties of dextrans and levans. Lack of correlation according to differences of molecular structure and size. Immunology. 1977 Aug;33(2):261–267. [PMC free article] [PubMed] [Google Scholar]
- Weber P. L., Gray G. R. Structural and immunochemical characterization of the acidic arabinomannan of Mycobacterium smegmatis. Carbohydr Res. 1979 Sep;74:259–278. doi: 10.1016/s0008-6215(00)84781-3. [DOI] [PubMed] [Google Scholar]
- Young D. B., Lamb J. R. T lymphocytes respond to solid-phase antigen: a novel approach to the molecular analysis of cellular immunity. Immunology. 1986 Oct;59(2):167–171. [PMC free article] [PubMed] [Google Scholar]
- Young D., Kent L., Rees A., Lamb J., Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986 Oct;54(1):177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


