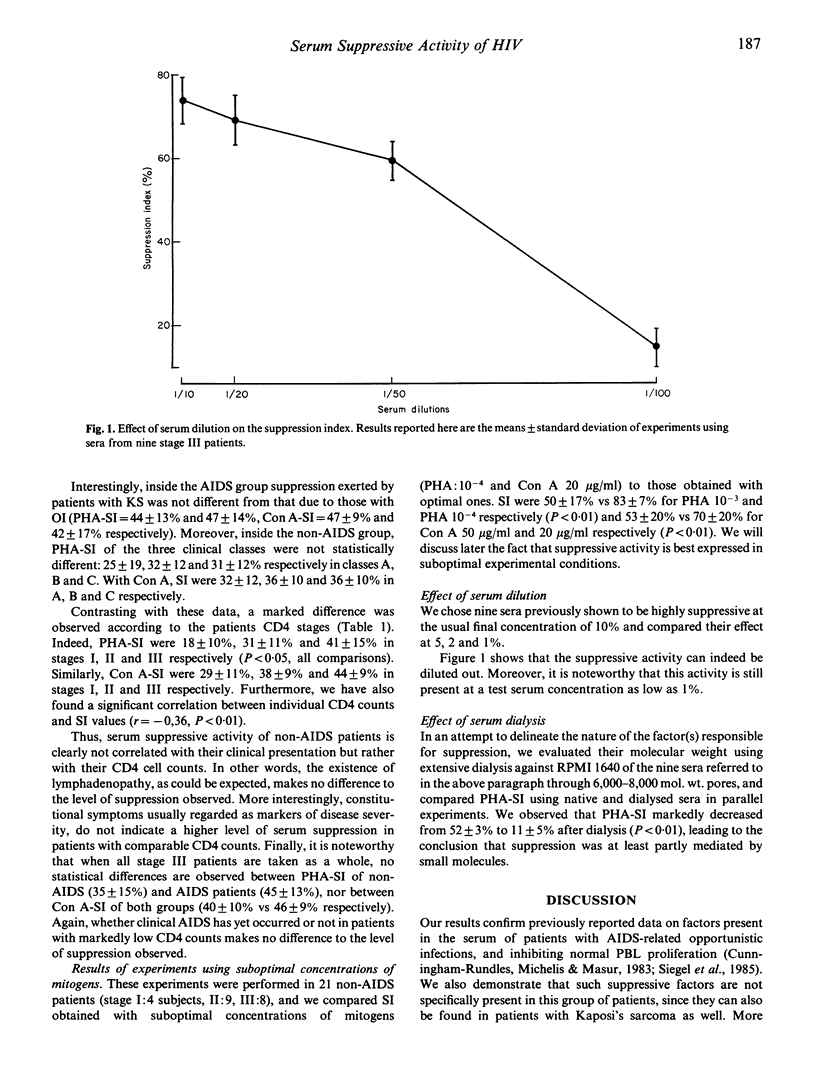
Abstract
The mechanisms by which HIV induces immunosuppression are still poorly understood so far. Several pathways of CD4 cell destruction are known, including cytolysis with or without syncitium formation and killing by cytotoxic effectors of HIV infected or non-infected CD4 cells. However, a discrepancy exists between the small number of actually infected cells in vivo and the extent of HIV-related immunodeficiency. Among other possible immunosuppressive factors, serum blocking factors have been reported, but only in AIDS-related opportunistic infections (OI), i.e. in a quite specific type of full-blown HIV disease. The purpose of this work was to determine whether serum blocking activity was unique to this group of patients, or if it was also expressed in other clinical presentations and, moreover, at earlier stages of the disease. We also attempted to delineate the nature of these seric factors. In order to do so, we assessed serum suppressive activity of 50 HIV seropositive patients, seven with OI, eight with Kaposi's sarcoma (KS), and 35 with no clinical AIDS. Our results confirm the existence of serum inhibiting factors in AIDS, and demonstrate their presence at earlier stages of the disease. They also highlight the fact that the level of serum suppression does not correlate with patients clinical status, but increases with the severity of the disease. The lower the CD4 count, the higher the suppression exerted. Furthermore, we showed that the suppression was at least partly mediated by small size molecules, which are not complement-mediated or directly lymphocytotoxic. On the other hand, this activity does not correlate with the serum level of p24 HIV core protein. The possible relation with other viral components is discussed. The relevance of these data to prognosis and pathogenesis of HIV disease deserves further investigation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amadori A., Faulkner-Valle G. P., De Rossi A., Zanovello P., Collavo D., Chieco-Bianchi L. HIV-mediated immunodepression: in vitro inhibition of T-lymphocyte proliferative response by ultraviolet-inactivated virus. Clin Immunol Immunopathol. 1988 Jan;46(1):37–54. doi: 10.1016/0090-1229(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Andrieu J. M., Even P., Venet A. AIDS and related syndromes as a viral-induced autoimmune disease of the immune system: an anti-MHC II disorder. Therapeutic implications. AIDS Res. 1986 Summer;2(3):163–174. doi: 10.1089/aid.1.1986.2.163. [DOI] [PubMed] [Google Scholar]
- Chanh T. C., Kennedy R. C., Kanda P. Synthetic peptides homologous to HIV transmembrane glycoprotein suppress normal human lymphocyte blastogenic response. Cell Immunol. 1988 Jan;111(1):77–86. doi: 10.1016/0008-8749(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Cianciolo G. J., Copeland T. D., Oroszlan S., Snyderman R. Inhibition of lymphocyte proliferation by a synthetic peptide homologous to retroviral envelope proteins. Science. 1985 Oct 25;230(4724):453–455. doi: 10.1126/science.2996136. [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles S., Michelis M. A., Masur H. Serum suppression of lymphocyte activation in vitro in acquired immunodeficiency disease. J Clin Immunol. 1983 Apr;3(2):156–165. doi: 10.1007/BF00915487. [DOI] [PubMed] [Google Scholar]
- Laurence J., Gottlieb A. B., Kunkel H. G. Soluble suppressor factors in patients with acquired immune deficiency syndrome and its prodrome. Elaboration in vitro by T lymphocyte-adherent cell interactions. J Clin Invest. 1983 Dec;72(6):2072–2081. doi: 10.1172/JCI111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence J., Mayer L. Immunoregulatory lymphokines of T hybridomas from AIDS patients: constitutive and inducible suppressor factors. Science. 1984 Jul 6;225(4657):66–69. doi: 10.1126/science.6328662. [DOI] [PubMed] [Google Scholar]
- Mann D. L., Lasane F., Popovic M., Arthur L. O., Robey W. G., Blattner W. A., Newman M. J. HTLV-III large envelope protein (gp120) suppresses PHA-induced lymphocyte blastogenesis. J Immunol. 1987 Apr 15;138(8):2640–2644. [PubMed] [Google Scholar]
- Orosz C. G., Zinn N. E., Olsen R. G., Mathes L. E. Retrovirus-mediated immunosuppression. I. FeLV-UV and specific FeLV proteins alter T lymphocyte behavior by inducing hyporesponsiveness to lymphokines. J Immunol. 1985 May;134(5):3396–3403. [PubMed] [Google Scholar]
- Ozturk G. E., Kohler P. F., Horsburgh C. R., Jr, Kirkpatrick C. H. The significance of antilymphocyte antibodies in patients with acquired immune deficiency syndrome (AIDS) and their sexual partners. J Clin Immunol. 1987 Mar;7(2):130–139. doi: 10.1007/BF00916007. [DOI] [PubMed] [Google Scholar]
- Pahwa S., Pahwa R., Saxinger C., Gallo R. C., Good R. A. Influence of the human T-lymphotropic virus/lymphadenopathy-associated virus on functions of human lymphocytes: evidence for immunosuppressive effects and polyclonal B-cell activation by banded viral preparations. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8198–8202. doi: 10.1073/pnas.82.23.8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Tanaka Y., Oda S., Chiba S., Suzuki H., Eto S., Yamashita U. Immunosuppressive factors from adult T-cell leukemia cells. Cancer Res. 1986 Sep;46(9):4458–4462. [PubMed] [Google Scholar]
- Siegel J. P., Djeu J. Y., Stocks N. I., Masur H., Gelmann E. P., Quinnan G. V., Jr Sera from patients with the acquired immunodeficiency syndrome inhibit production of interleukin-2 by normal lymphocytes. J Clin Invest. 1985 Jun;75(6):1957–1964. doi: 10.1172/JCI111912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Campbell K., Haseltine W. A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. 1986 Jul 31-Aug 6Nature. 322(6078):470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- Somasundaran M., Robinson H. L. A major mechanism of human immunodeficiency virus-induced cell killing does not involve cell fusion. J Virol. 1987 Oct;61(10):3114–3119. doi: 10.1128/jvi.61.10.3114-3119.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker R. B., McHugh T. M., Moody D. J., Morrow W. J., Stites D. P., Shuman M. A., Levy J. A. An AIDS-related cytotoxic autoantibody reacts with a specific antigen on stimulated CD4+ T cells. 1987 Jun 25-Jul 1Nature. 327(6124):710–713. doi: 10.1038/327710a0. [DOI] [PubMed] [Google Scholar]
- Sugden P. J., Lilleyman J. S. Impairment of lymphocyte transformation by plasma from patients with advanced Hodgkin's disease. Cancer. 1980 Mar 1;45(5):899–905. doi: 10.1002/1097-0142(19800301)45:5<899::aid-cncr2820450512>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Tas M., Drexhage H. A., Goudsmit J. A monocyte chemotaxis inhibiting factor in serum of HIV infected men shares epitopes with the HIV transmembrane protein gp41. Clin Exp Immunol. 1988 Jan;71(1):13–18. [PMC free article] [PubMed] [Google Scholar]
- Wainberg M. A., Blain N., Spira B. Inhibition of human lymphocyte mitogenesis by human and other retroviruses. Differential effect of interleukin-2 in restoration of responsiveness. AIDS. 1987 Jul;1(2):83–87. [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Ziegler J. L., Stites D. P. Hypothesis: AIDS is an autoimmune disease directed at the immune system and triggered by a lymphotropic retrovirus. Clin Immunol Immunopathol. 1986 Dec;41(3):305–313. doi: 10.1016/0090-1229(86)90001-2. [DOI] [PubMed] [Google Scholar]


