Abstract
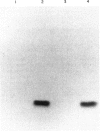
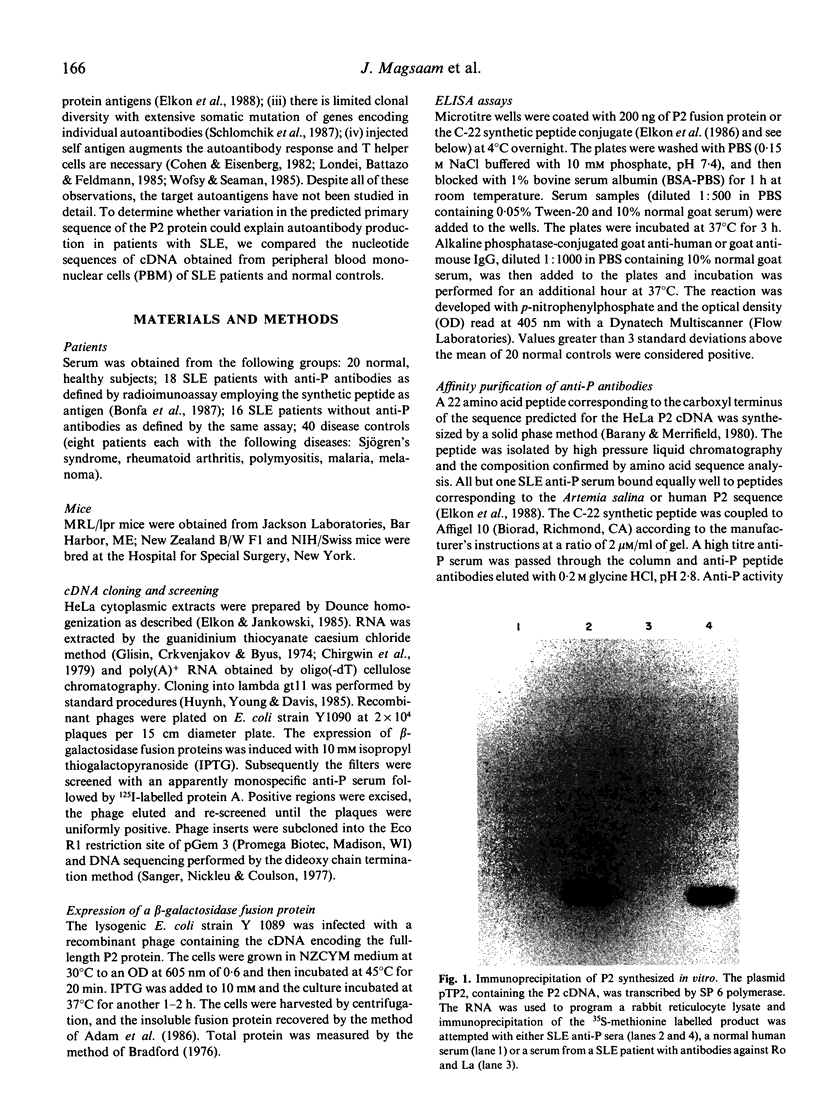
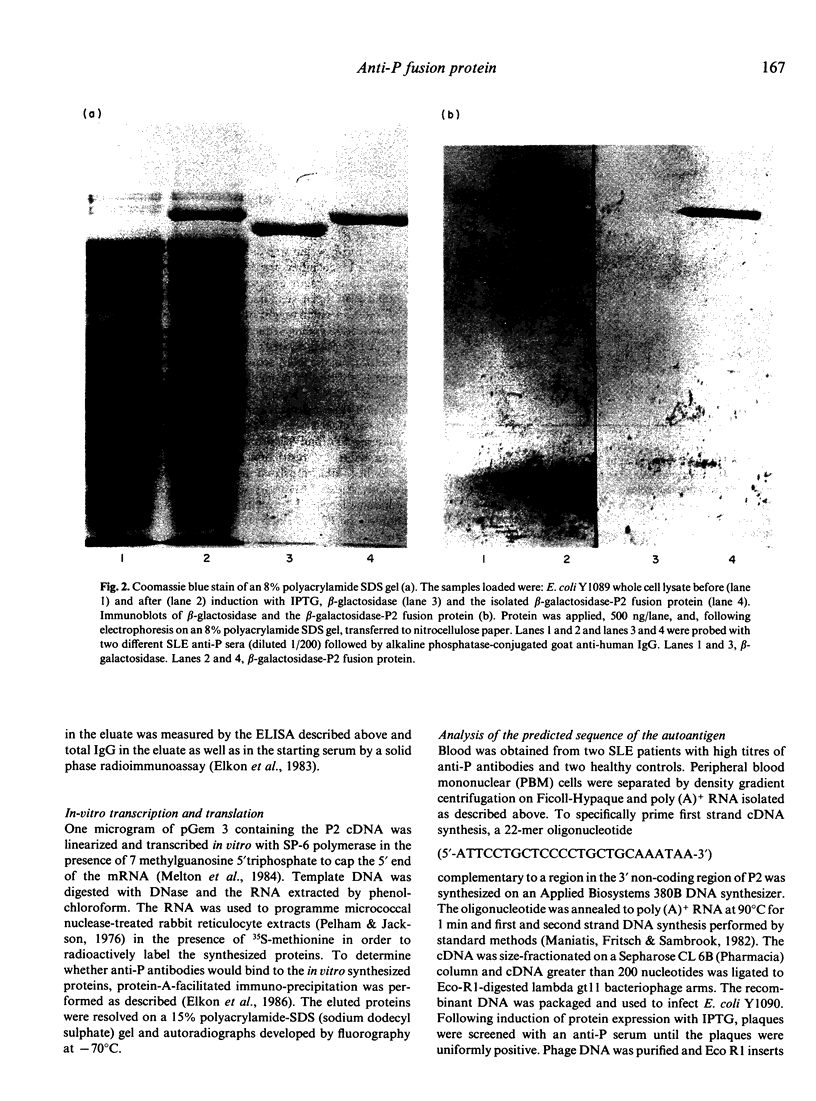
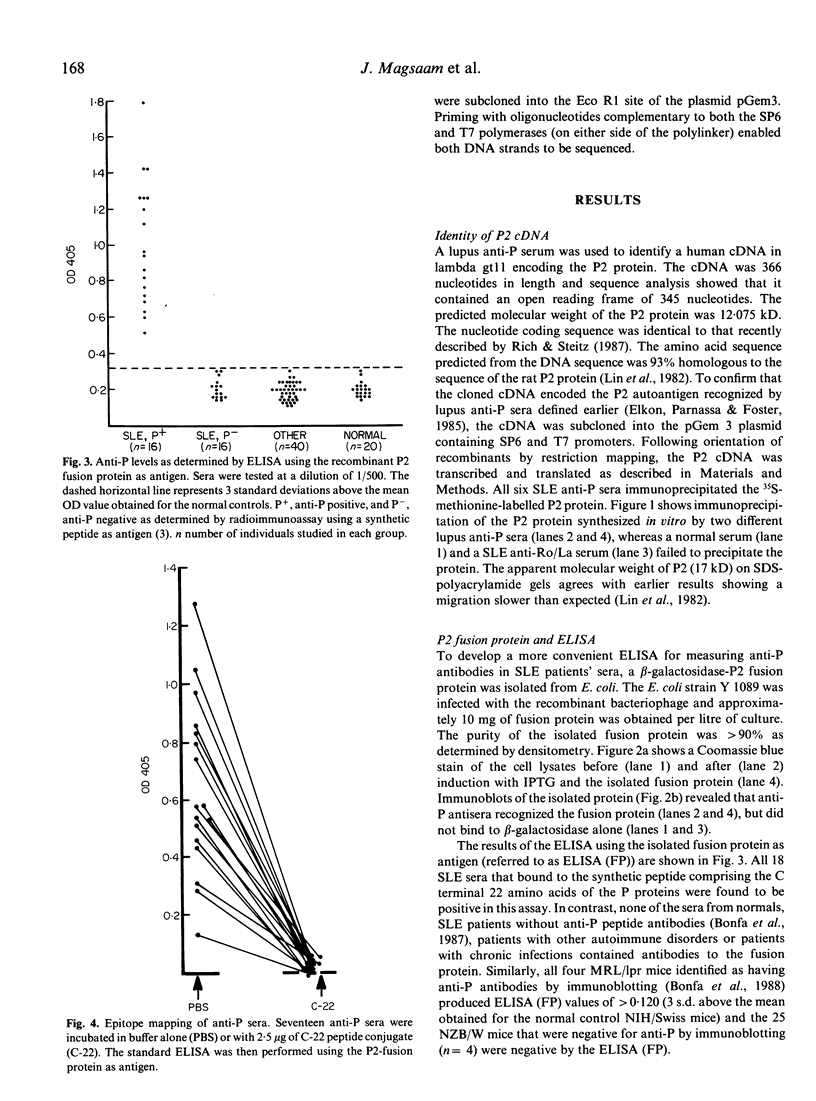
The cDNA encoding the ribosomal protein P2 antigen was cloned from a human cDNA library constructed in the lambda gt11 expression vector. A beta-galactosidase-P2 fusion protein was purified to near homogeneity and used to develop an ELISA which was highly specific for anti-P antibodies produced in murine and human SLE. The median concentration of human IgG anti-P antibodies in serum was estimated to be 100 micrograms/ml (range 6-450 micrograms/ml). Pre-incubation of human anti-P sera with a synthetic peptide, corresponding to the C-terminal 22 amino acids of P2, completely inhibited reactivity with the fusion protein in the ELISA. These findings confirm that lupus anti-P sera show a striking restriction in epitope specificity and indicate that the P2 fusion protein is a useful alternative to the synthetic peptide antigen for detection and quantification of anti-P antibodies. To investigate the possibility that anti-P antibodies were induced by 'altered-self', cDNA encoding P2 were also cloned from lupus patients and control mononuclear cells. The predicted amino acid sequences of the patients' P2 were identical to that of the normal controls indicating that a primary structural abnormality of the P2 autoantigen was unlikely.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Nakagawa T., Swanson M. S., Woodruff T. K., Dreyfuss G. mRNA polyadenylate-binding protein: gene isolation and sequencing and identification of a ribonucleoprotein consensus sequence. Mol Cell Biol. 1986 Aug;6(8):2932–2943. doi: 10.1128/mcb.6.8.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Denman A. M., Barnes R. D. Cooperating and controlling functions of thymus-derived lymphocytes in relation to autoimmunity. Lancet. 1971 Jul 17;2(7716):135–140. doi: 10.1016/s0140-6736(71)92306-3. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Elkon K. B. Clinical and serologic associations of the antiribosomal P protein antibody. Arthritis Rheum. 1986 Aug;29(8):981–985. doi: 10.1002/art.1780290806. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Golombek S. J., Kaufman L. D., Skelly S., Weissbach H., Brot N., Elkon K. B. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987 Jul 30;317(5):265–271. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Marshak-Rothstein A., Weissbach H., Brot N., Elkon K. Frequency and epitope recognition of anti-ribosome P antibodies from humans with systemic lupus erythematosus and MRL/lpr mice are similar. J Immunol. 1988 May 15;140(10):3434–3437. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christian C. L., Elkon K. B. Autoantibodies to intracellular proteins. Clinical and biologic significance. Am J Med. 1986 Jan;80(1):53–61. doi: 10.1016/0002-9343(86)90048-3. [DOI] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. T cell recognition of the Sm nuclear antigen: induction of T cell proliferative responses in MRL/Mp- +/+ mice. J Immunol. 1982 Nov;129(5):2142–2145. [PubMed] [Google Scholar]
- Earnshaw W. C., Machlin P. S., Bordwell B. J., Rothfield N. F., Cleveland D. W. Analysis of anticentromere autoantibodies using cloned autoantigen CENP-B. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4979–4983. doi: 10.1073/pnas.84.14.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Gharavi A. E., Patel B. M., Hughes G. R., Frankel A. IgA and IgM rheumatoid factors in serum, saliva and other secretions: relationship to immunoglobulin ratios in systemic sicca syndrome and rheumatoid arthritis. Clin Exp Immunol. 1983 Apr;52(1):75–84. [PMC free article] [PubMed] [Google Scholar]
- Elkon K. B., Jankowski P. W. Fine specificities of autoantibodies directed against the Ro, La, Sm, RNP, and Jo-1 proteins defined by two-dimensional gel electrophoresis and immunoblotting. J Immunol. 1985 Jun;134(6):3819–3824. [PubMed] [Google Scholar]
- Elkon K. B., Parnassa A. P., Foster C. L. Lupus autoantibodies target ribosomal P proteins. J Exp Med. 1985 Aug 1;162(2):459–471. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K., Bonfa E., Llovet R., Danho W., Weissbach H., Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5186–5189. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon K., Skelly S., Parnassa A., Moller W., Danho W., Weissbach H., Brot N. Identification and chemical synthesis of a ribosomal protein antigenic determinant in systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7419–7423. doi: 10.1073/pnas.83.19.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharavi A. E., Chu J. L., Elkon K. B. Autoantibodies to intracellular proteins in human systemic lupus erythematosus are not due to random polyclonal B cell activation. Arthritis Rheum. 1988 Nov;31(11):1337–1345. doi: 10.1002/art.1780311101. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Wittmann-Liebold B., McNally J., Wool I. G. The primary structure of the acidic phosphoprotein P2 from rat liver 60 S ribosomal subunits. Comparison with ribosomal 'A' proteins from other species. J Biol Chem. 1982 Aug 10;257(15):9189–9197. [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Maddison P. J., Reichlin M. Quantitation of precipitating antibodies to certain soluble nuclear antigens in SLE. Arthritis Rheum. 1977 Apr;20(3):819–824. doi: 10.1002/art.1780200310. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netter H. J., Guldner H. H., Szostecki C., Lakomek H. J., Will H. A recombinant autoantigen derived from the human (U1) small nuclear RNP-specific 68-kd protein. Expression in Escherichia coli and serodiagnostic application. Arthritis Rheum. 1988 May;31(5):616–622. doi: 10.1002/art.1780310506. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pontes de Carvalho L. C., Templeman J., Wick G., Roitt I. M. The role of self-antigen in the development of autoimmunity in Obese strain chickens with spontaneous autoallergic thyroiditis. J Exp Med. 1982 May 1;155(5):1255–1266. doi: 10.1084/jem.155.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich B. E., Steitz J. A. Human acidic ribosomal phosphoproteins P0, P1, and P2: analysis of cDNA clones, in vitro synthesis, and assembly. Mol Cell Biol. 1987 Nov;7(11):4065–4074. doi: 10.1128/mcb.7.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M. J., Marshak-Rothstein A., Wolfowicz C. B., Rothstein T. L., Weigert M. G. The role of clonal selection and somatic mutation in autoimmunity. 1987 Aug 27-Sep 2Nature. 328(6133):805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- St Clair E. W., Pisetsky D. S., Reich C. F., Chambers J. C., Keene J. D. Quantitative immunoassay of anti-La antibodies using purified recombinant La antigen. Arthritis Rheum. 1988 Apr;31(4):506–514. doi: 10.1002/art.1780310407. [DOI] [PubMed] [Google Scholar]
- Sundick R. S., Wick G. Increased iodine uptake by obese strain thyroid glands transplanted to normal chick embryos. J Immunol. 1976 May;116(5):1319–1323. [PubMed] [Google Scholar]
- TOWNES A. S., STEWART C. R., Jr, OSLER A. G. Immunologic studies of systemic lupus erythematosus. II. Variations of nucleoprotein-reactive gamma globulin and hemolytic serum complement levels with disease activity. Bull Johns Hopkins Hosp. 1963 Apr;112:202–219. [PubMed] [Google Scholar]
- Truden J. L., Sundick R. S., Levine S., Rose N. R. The decreased growth rate of obese strain chicken thyroid cells provides in vitro evidence for a primary target organ abnormality in chickens susceptible to autoimmune thyroiditis. Clin Immunol Immunopathol. 1983 Nov;29(2):294–305. doi: 10.1016/0090-1229(83)90031-4. [DOI] [PubMed] [Google Scholar]
- Uchiumi T., Wahba A. J., Traut R. R. Topography and stoichiometry of acidic proteins in large ribosomal subunits from Artemia salina as determined by crosslinking. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5580–5584. doi: 10.1073/pnas.84.16.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITEBSKY E., ROSE N. R. Studies on organ specificity. IV. Production of rabbit thyroid antibodies in the rabbit. J Immunol. 1956 Jun;76(6):408–416. [PubMed] [Google Scholar]
- Weigle W. O. The production of thyroiditis and antibody following injection of unaltered thyroglobulin without adjuvant into rabbits previously stimulated with altered thyroglobulin. J Exp Med. 1965 Dec 1;122(6):1049–1062. doi: 10.1084/jem.122.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]