Abstract
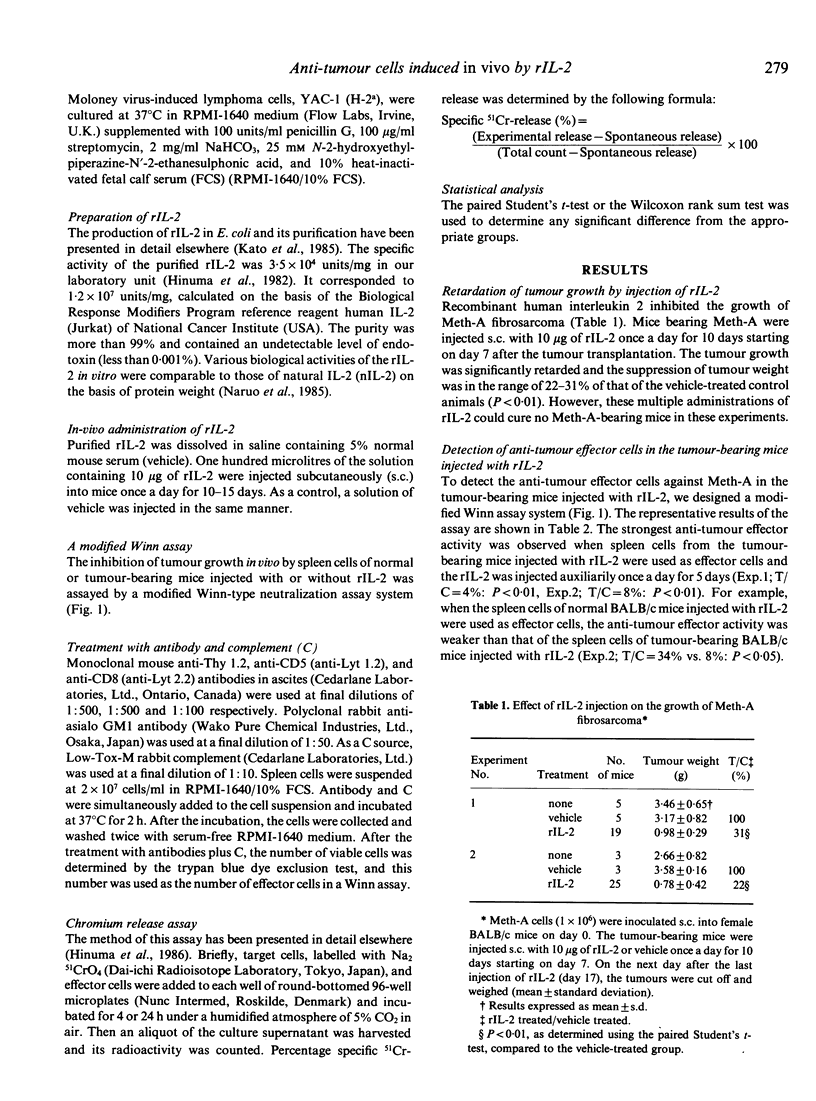
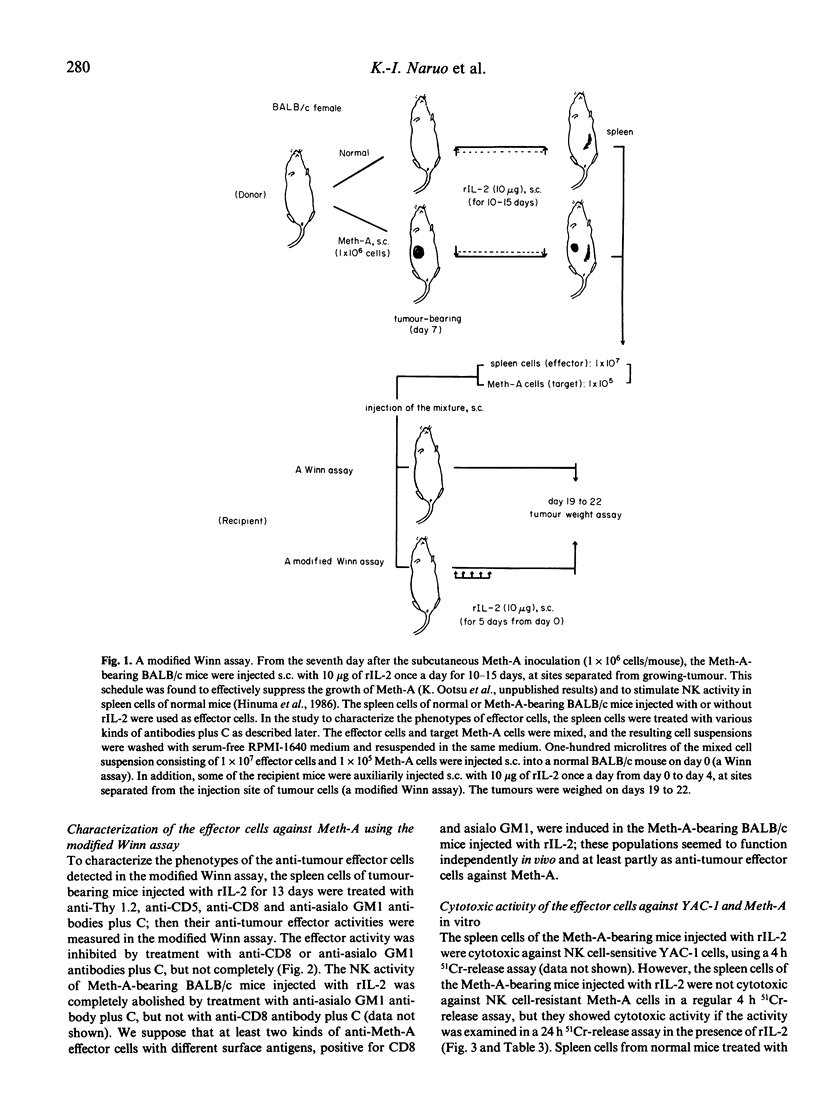
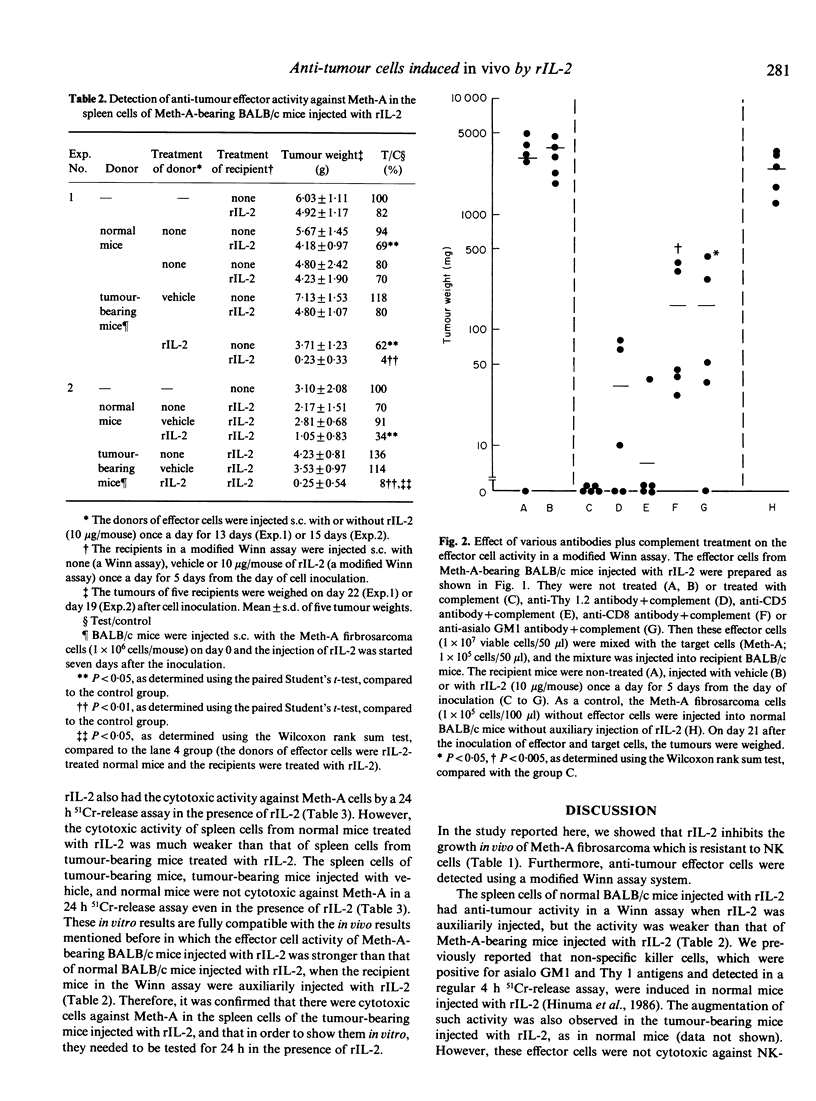
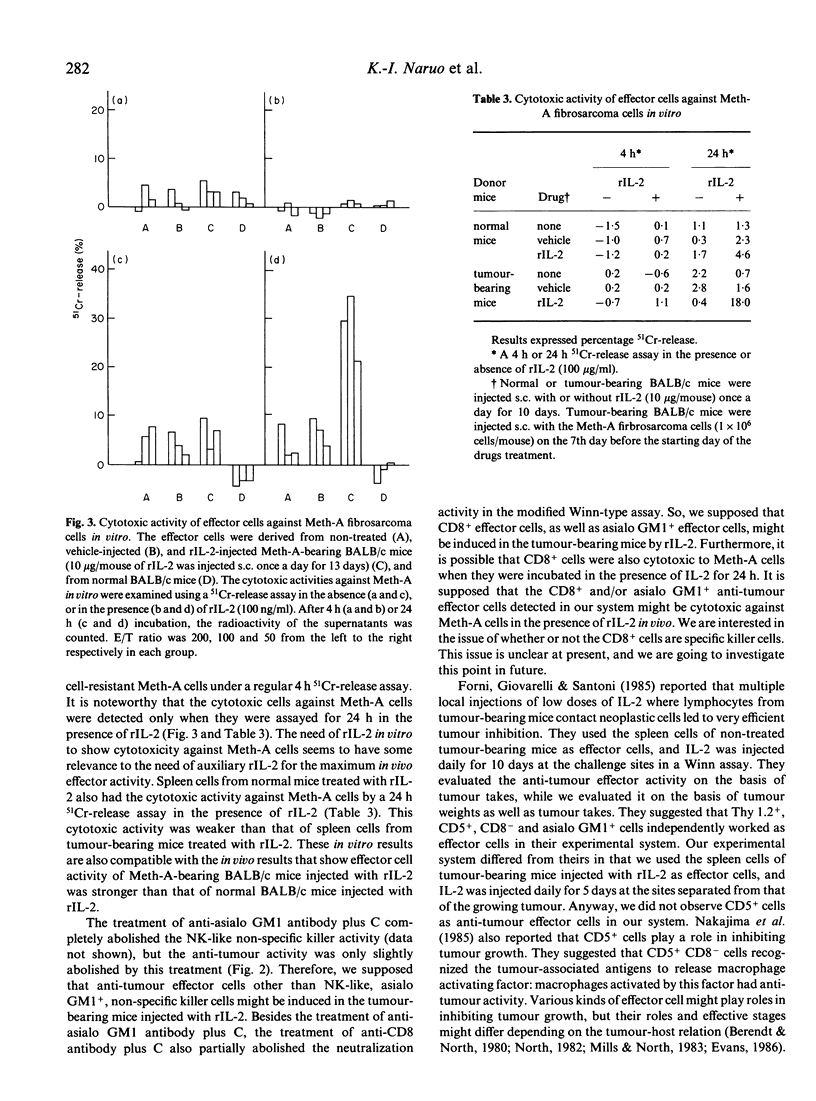
When Meth-A fibrosarcoma-bearing BALB/c mice were injected subcutaneously with 10 micrograms of recombinant human interleukin 2(rIL-2) once a day for 10 days, tumour growth inhibition was in the range of 22-31% of that of the control animals. Anti-tumour effector cells against Meth-A were detected in the spleen cells of the tumour-bearing BALB/c mice injected with rIL-2, using a modified Winn-type neutralization assay with the auxiliary injection of rIL-2. To induce the strongest anti-tumour activity in this assay system, the following were necessary: 1) the effector cells were derived from tumour-bearing BALB/c mice; 2) the donors of the effector cells were injected with rIL-2; 3) the recipient mice in the Winn assay were auxiliarily injected with rIL-2 (a modified Winn assay). The anti-tumour effector activity detected in the modified Winn assay was inhibited by treatment with anti-CD8 or anti-asialo GM1 antibodies plus complement (C), but not completely. We supposed that at least two kinds of anti-Meth-A effector cells with different surface antigens, positive for CD8 and asialo GM1 antigens, were induced in the Meth-A-bearing BALB/c mice injected with rIL-2; these populations seemed to function independently and at least partly as anti-tumour effector cells in this tumour-host system. These spleen cells showed in vitro cytotoxicity against Meth-A cells, which are resistant to NK cells, if the activity was measured in a 24 h 51Cr-release assay in the presence of rIL-2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berendt M. J., North R. J. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med. 1980 Jan 1;151(1):69–80. doi: 10.1084/jem.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeník J., Perlmann P., Indrová M., Símová J., Jandlová T., Neuwirt J. Growth inhibition of an MC-induced mouse sarcoma by TCGF (IL 2)-containing preparations. Preliminary report. Cancer Immunol Immunother. 1983;14(3):205–206. doi: 10.1007/BF00205362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. E., Hyatt C. L., Rosenberg S. A. Systemic administration of recombinant human interleukin-2 in mice. J Biol Response Mod. 1984 Oct;3(5):561–572. [PubMed] [Google Scholar]
- Evans R. The immunological network at the site of tumor rejection. Biochim Biophys Acta. 1986 Aug 5;865(1):1–11. doi: 10.1016/0304-419x(86)90009-0. [DOI] [PubMed] [Google Scholar]
- Forni G., Giovarelli M., Santoni A. Lymphokine-activated tumor inhibition in vivo. I. The local administration of interleukin 2 triggers nonreactive lymphocytes from tumor-bearing mice to inhibit tumor growth. J Immunol. 1985 Feb;134(2):1305–1311. [PubMed] [Google Scholar]
- Hinuma S., Naruo K., Ootsu K., Houkan T., Shiho O., Tsukamoto K. Suppression of pulmonary tumour metastasis in mice by recombinant human interleukin-2: role of asialo GM1-positive cells. Immunology. 1987 Feb;60(2):173–179. [PMC free article] [PubMed] [Google Scholar]
- Hinuma S., Naruo K., Shiho O., Tsukamoto K. Characteristics of murine non-specific killer cells induced in vivo by recombinant human interleukin-2. Immunology. 1986 Oct;59(2):251–259. [PMC free article] [PubMed] [Google Scholar]
- Hinuma S., Onda H., Naruo K., Ichimori Y., Koyama M., Tsukamoto K. Translation of interleukin 2 mRNA from human peripheral blood leukocytes in Xenopus oocytes. Biochem Biophys Res Commun. 1982 Nov 30;109(2):363–369. doi: 10.1016/0006-291x(82)91729-6. [DOI] [PubMed] [Google Scholar]
- Kato K., Yamada T., Kawahara K., Onda H., Asano T., Sugino H., Kakinuma A. Purification and characterization of recombinant human interleukin-2 produced in Escherichia coli. Biochem Biophys Res Commun. 1985 Jul 31;130(2):692–699. doi: 10.1016/0006-291x(85)90472-3. [DOI] [PubMed] [Google Scholar]
- Mazumder A., Rosenberg S. A. Successful immunotherapy of natural killer-resistant established pulmonary melanoma metastases by the intravenous adoptive transfer of syngeneic lymphocytes activated in vitro by interleukin 2. J Exp Med. 1984 Feb 1;159(2):495–507. doi: 10.1084/jem.159.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills C. D., North R. J. Expression of passively transferred immunity against an established tumor depends on generation of cytolytic T cells in recipient. Inhibition by suppressor T cells. J Exp Med. 1983 May 1;157(5):1448–1460. doi: 10.1084/jem.157.5.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Nakajima H., Fujiwara H., Takai Y., Izumi Y., Sano S., Tsuchida T., Hamaoka T. Studies on macrophage-activating factor (MAF) in antitumor immune responses. I. Tumor-specific Lyt-1+2- T cells are required for producing MAF able to generate cytolytic as well as cytostatic macrophages. J Immunol. 1985 Sep;135(3):2199–2205. [PubMed] [Google Scholar]
- Naruo K., Hinuma S., Kato K., Koyama M., Tada H., Shiho O., Tsukamoto K. Comparison of the biological properties of purified natural and recombinant human interleukin-2. Biochem Biophys Res Commun. 1985 Apr 16;128(1):257–264. doi: 10.1016/0006-291x(85)91672-9. [DOI] [PubMed] [Google Scholar]
- North R. J. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982 Apr 1;155(4):1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Mulé J. J., Spiess P. J., Reichert C. M., Schwarz S. L. Regression of established pulmonary metastases and subcutaneous tumor mediated by the systemic administration of high-dose recombinant interleukin 2. J Exp Med. 1985 May 1;161(5):1169–1188. doi: 10.1084/jem.161.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]


