Abstract
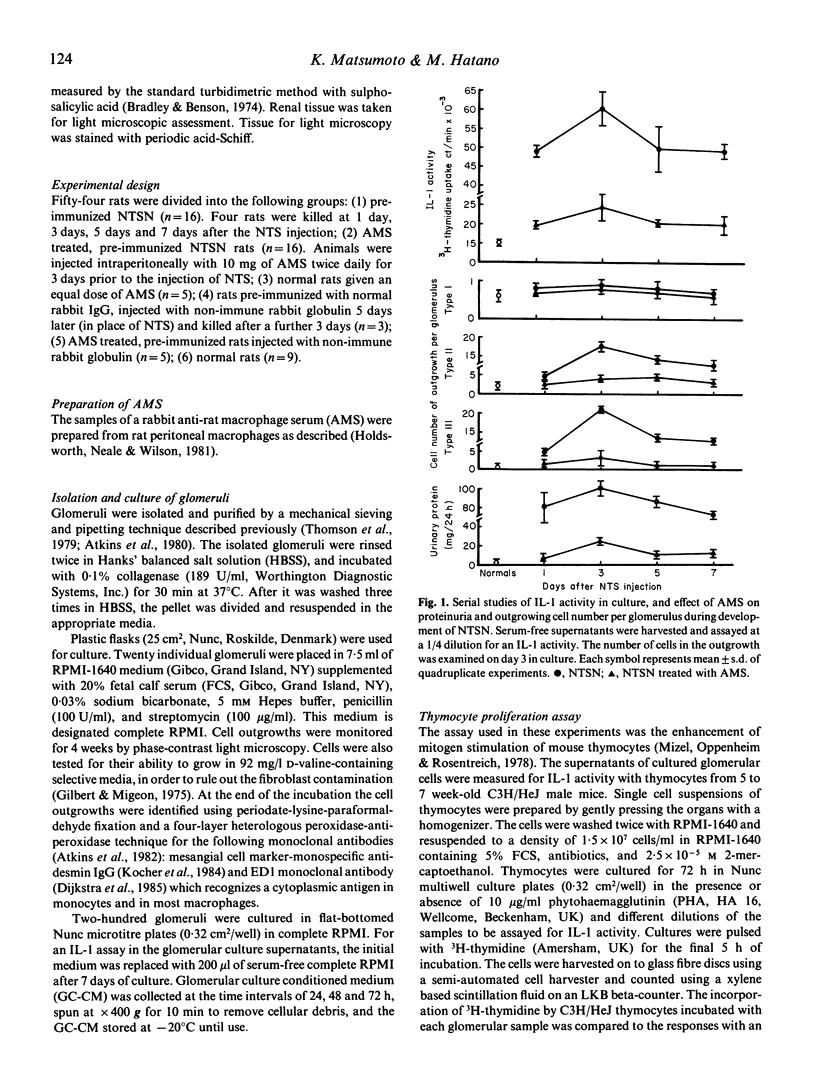
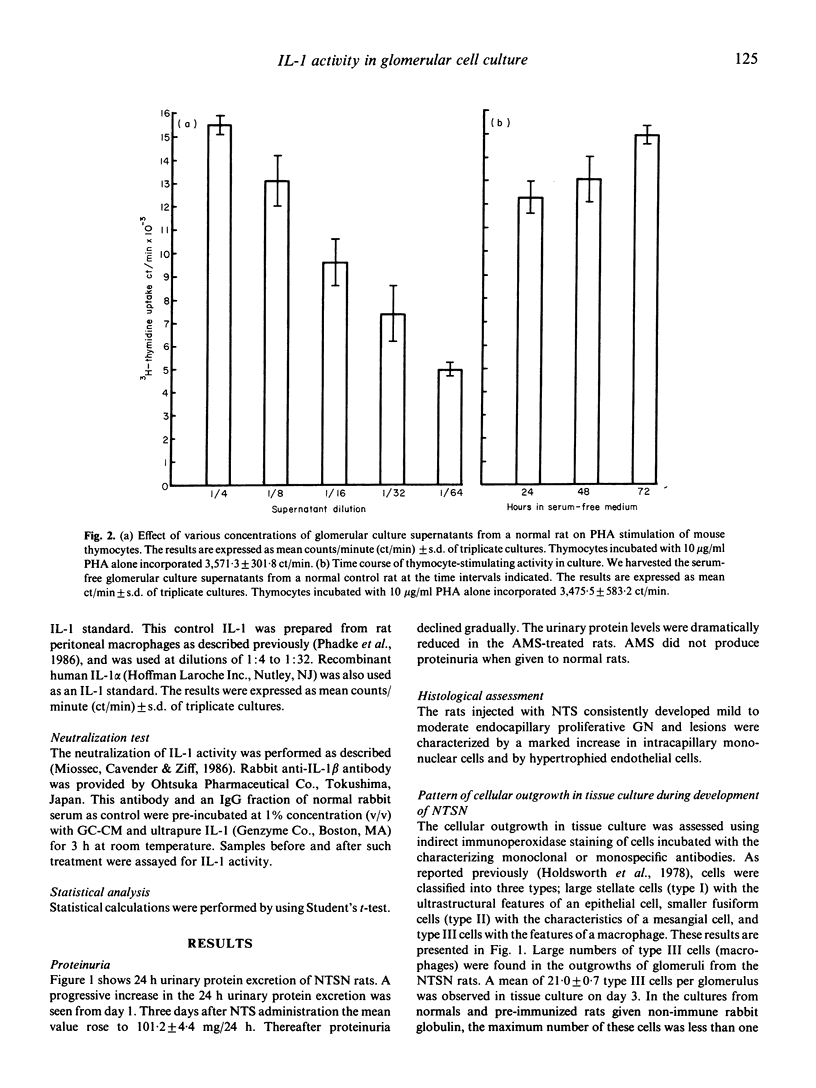
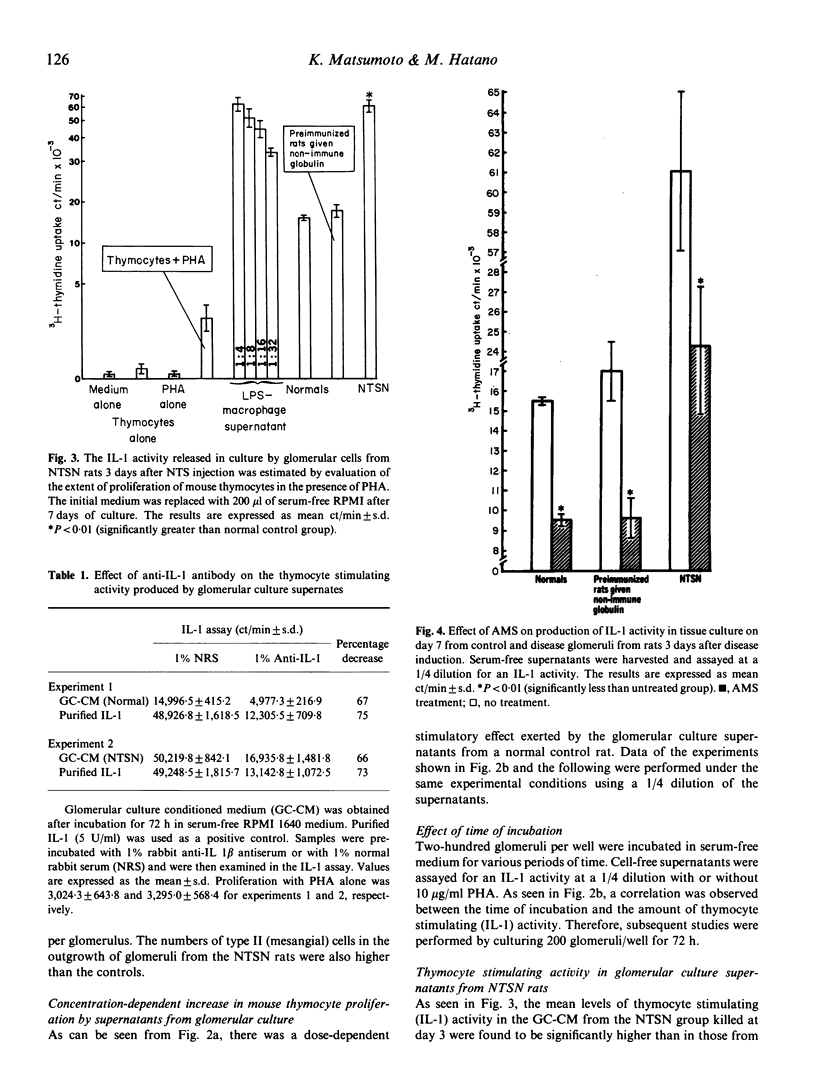
To investigate the role of cytokines in the pathogenesis of experimental glomerulonephritis (GN), we examined interleukin 1 (IL-1) activity in isolated glomeruli of rats with an accelerated autologous form of nephrotoxic serum nephritis (NTSN). Glomeruli were isolated and explanted in tissue culture. The prominent feature of the glomerular outgrowth of the glomeruli in the NTSN was the presence of large numbers of type III cells (macrophages). In addition, there were significantly greater numbers of type II (mesangial) cells in culture from the NTSN rats as compared with glomeruli from normal rats, though the numbers of type I (epithelial) cells were the same. IL-1 bioactivity was significantly higher in culture supernatants generated by glomeruli isolated from NTSN animals versus normal animals early in the course of the disease. When glomerular culture supernatants and purified IL-1 were pre-incubated with an anti-IL-1 beta antibody, a parallel decrease of the biological activity was found, suggesting that the biologically active binding sites of the culture supernatants and monocyte-produced IL-1 share some common structure. The administration of a rabbit anti-rat macrophage serum (AMS) prevented the outgrowths of type III cells (macrophages) and type II (mesangial) cells and reduced the production of IL-1 activity in the NTSN rats. In this experimental model IL-1 synthesis, probably by multiple cell types, is present early in the disease process and perhaps may be an important mediator of glomerular immune injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins R. C., Glasgow E. F., Holdsworth S. R., Thomson N. M., Hancock W. W. Tissue culture of isolated glomeruli from patients with glomerulonephritis. Kidney Int. 1980 Apr;17(4):515–527. doi: 10.1038/ki.1980.60. [DOI] [PubMed] [Google Scholar]
- Atkins R. C., Holdsworth S. R., Glasgow E. F., Matthews F. E. The macrophagen in human rapidly progressive glomerulonephritis. Lancet. 1976 Apr 17;1(7964):830–832. doi: 10.1016/s0140-6736(76)90480-3. [DOI] [PubMed] [Google Scholar]
- Atkins R. C., Holdsworth S. R., Hancock W. W., Thomson N. M., Glasgow E. F. Cellular immune mechanisms in human glomerulonephritis: the role of mononuclear leucocytes. Springer Semin Immunopathol. 1982;5(3):269–296. doi: 10.1007/BF01892089. [DOI] [PubMed] [Google Scholar]
- Beck G., Habicht G. S., Benach J. L., Miller F. Interleukin 1: a common endogenous mediator of inflammation and the local Shwartzman reaction. J Immunol. 1986 Apr 15;136(8):3025–3031. [PubMed] [Google Scholar]
- Bhan A. K., Schneeberger E. E., Collins A. B., McCluskey R. T. Evidence for a pathogenic role of a cell-mediated immune mechanism in experimental glomerulonephritis. J Exp Med. 1978 Jul 1;148(1):246–260. doi: 10.1084/jem.148.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985 Mar;54(3):589–599. [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. An update on human interleukin-1: from molecular biology to clinical relevance. J Clin Immunol. 1985 Sep;5(5):287–297. doi: 10.1007/BF00918247. [DOI] [PubMed] [Google Scholar]
- Gilbert S. F., Migeon B. R. D-valine as a selective agent for normal human and rodent epithelial cells in culture. Cell. 1975 May;5(1):11–17. doi: 10.1016/0092-8674(75)90086-0. [DOI] [PubMed] [Google Scholar]
- Holdsworth S. R., Glasgow E. F., Atkins R. C., Thomson N. M. Cell characteristics of cultured glomeruli from different animal species. Nephron. 1978;22(4-6):454–459. doi: 10.1159/000181513. [DOI] [PubMed] [Google Scholar]
- Holdsworth S. R., Neale T. J., Wilson C. B. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest. 1981 Sep;68(3):686–698. doi: 10.1172/JCI110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Lovett D. H., Ryan J. L., Sterzel R. B. A thymocyte-activating factor derived from glomerular mesangial cells. J Immunol. 1983 Apr;130(4):1796–1801. [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Ooi B. S., MacCarthy E. P., Hsu A., Ooi Y. M. Human mononuclear cell modulation of endothelial cell proliferation. J Lab Clin Med. 1983 Sep;102(3):428–433. [PubMed] [Google Scholar]
- Phadke K., Carlson D. G., Gitter B. D., Butler L. D. Role of interleukin 1 and interleukin 2 in rat and mouse arthritis models. J Immunol. 1986 Jun 1;136(11):4085–4091. [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Schreiner G. F., Cotran R. S., Pardo V., Unanue E. R. A mononuclear cell component in experimental immunological glomerulonephritis. J Exp Med. 1978 Feb 1;147(2):369–384. doi: 10.1084/jem.147.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson N. M., Holdsworth S. R., Glasgow E. F., Atkins R. C. The macrophage in the development of experimental crescentic glomerulonephritis. Studies using tissue culture and electron microscopy. Am J Pathol. 1979 Feb;94(2):223–240. [PMC free article] [PubMed] [Google Scholar]
- Werber H. I., Emancipator S. N., Tykocinski M. L., Sedor J. R. The interleukin 1 gene is expressed by rat glomerular mesangial cells and is augmented in immune complex glomerulonephritis. J Immunol. 1987 May 15;138(10):3207–3212. [PubMed] [Google Scholar]


