Abstract
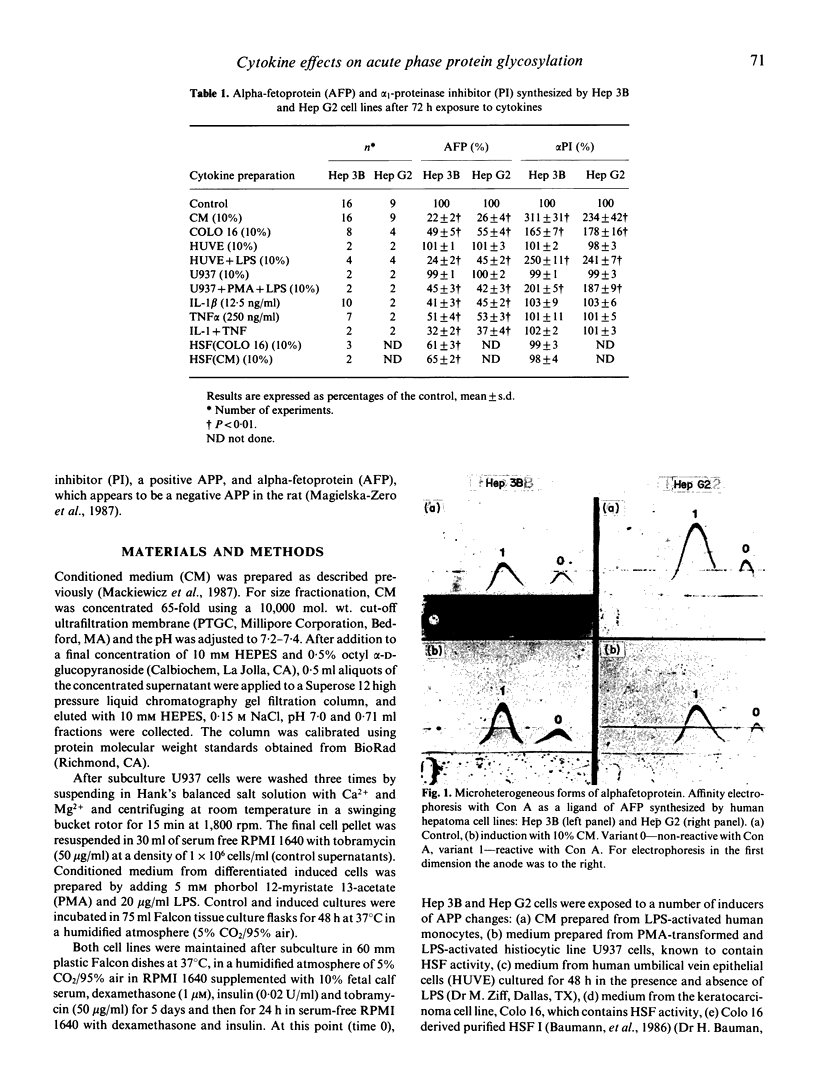
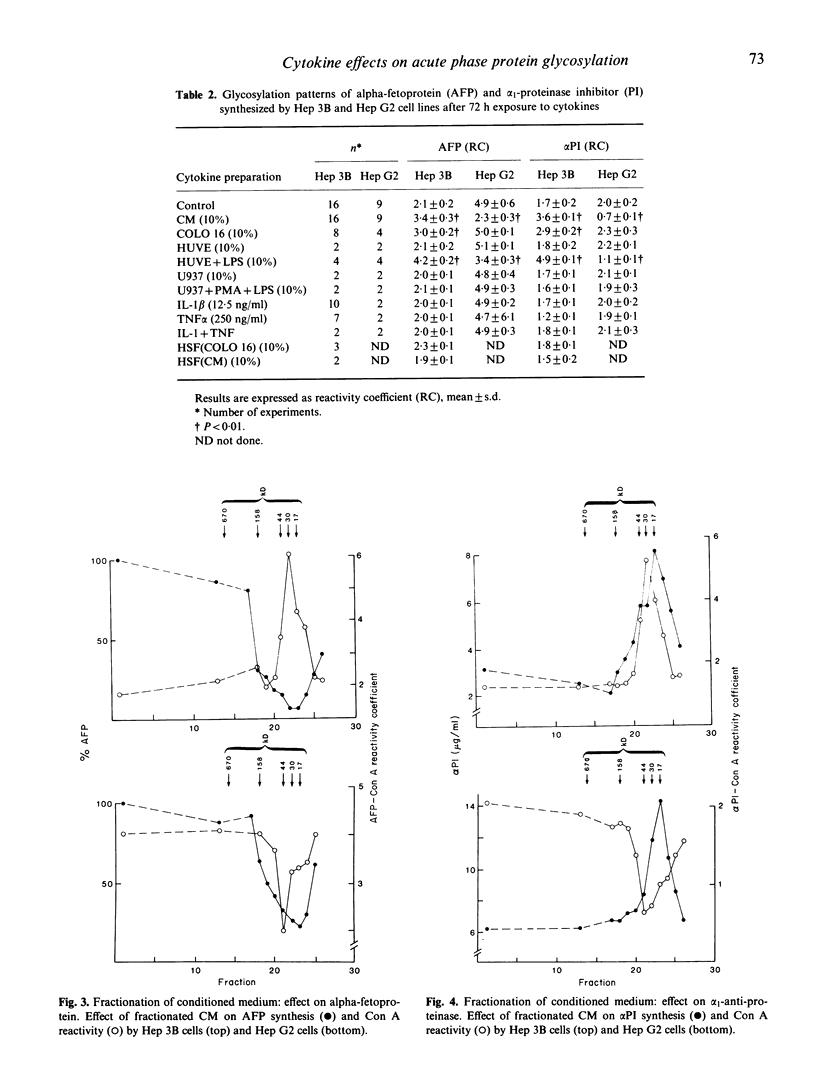
The effects of various cytokines on synthesis and microheterogeneity of carbohydrate structure of alpha 1-proteinase inhibitor (PI) and alpha-fetoprotein (AFP) in the human hepatoma cell lines Hep 3B and Hep G2 were studied. In both lines, crude cytokine preparations from LPS-activated human monocytes (CM) and several cell lines led to increased PI and decreased AFP synthesis, while recombinant interleukin 1 (IL-1), recombinant tumor necrosis factor (TNF) and hepatocyte stimulating factor preparations (HSF) affected AFP but not PI production. Several of the crude cytokine preparations, but not IL-1, TNF, or HSF, caused Hep 3B cells to secrete forms of PI and AFP showing increased reactivity with Con A upon testing by affinity electrophoresis, while decreased reactivity with Con A was seen in these proteins secreted by Hep G2 cells. Determination of molecular size of PI inducing activity in CM showed a sharp peak at about 17 kD while AFP inhibiting activity was present in a very broad range of molecular size fractions maximal at 17-30 kD. Changes in patterns of glycosylation of these proteins were attributable to cytokines of about 30 kD in Hep 3B and 44 kD in Hep G2 cells. These findings demonstrate the existence of a family of glycosylation regulating cytokines, and suggest that distinct mechanisms within hepatocytes, responsive to different cytokines, may lead to increased or decreased Con A binding of glycoproteins and to altered gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Hill R. E., Sauder D. N., Jahreis G. P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986 Feb;102(2):370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bog-Hansen T. C. Crossed immuno-affinoelectrophoresis. An analytical method to predict the result of affinity chromatography. Anal Biochem. 1973 Dec;56(2):480–488. doi: 10.1016/0003-2697(73)90215-7. [DOI] [PubMed] [Google Scholar]
- Carlson J., Eriksson S., Alm R., Kjellström T. Biosynthesis of abnormally glycosylated alpha 1-antitrypsin by a human hepatoma cell line. Hepatology. 1984 Mar-Apr;4(2):235–241. doi: 10.1002/hep.1840040211. [DOI] [PubMed] [Google Scholar]
- Ganapathi M. K., Schultz D., Mackiewicz A., Samols D., Hu S. I., Brabenec A., Macintyre S. S., Kushner I. Heterogeneous nature of the acute phase response. Differential regulation of human serum amyloid A, C-reactive protein, and other acute phase proteins by cytokines in Hep 3B cells. J Immunol. 1988 Jul 15;141(2):564–569. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger G., Arnaout M. A., Aden D., Kay R., Rits M., Colten H. R. Biosynthesis and postsynthetic processing of human C3b/C4b inactivator (factor I) in three hepatoma cell lines. J Biol Chem. 1984 May 25;259(10):6492–6497. [PubMed] [Google Scholar]
- Kushner I., Mackiewicz A. Acute phase proteins as disease markers. Dis Markers. 1987 Mar;5(1):1–11. [PubMed] [Google Scholar]
- Laurell C. B. Electroimmuno assay. Scand J Clin Lab Invest Suppl. 1972;124:21–37. doi: 10.3109/00365517209102748. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A., Ganapathi M. K., Schultz D., Kushner I. Monokines regulate glycosylation of acute-phase proteins. J Exp Med. 1987 Jul 1;166(1):253–258. doi: 10.1084/jem.166.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackiewicz A., Mackiewicz S. Determination of lectin-sugar dissociation constants by agarose affinity electrophoresis. Anal Biochem. 1986 Aug 1;156(2):481–488. doi: 10.1016/0003-2697(86)90282-4. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A., Marcinkowska-Pieta R., Ballou S., Mackiewicz S., Kushner I. Microheterogeneity of alpha 1-acid glycoprotein in the detection of intercurrent infection in systemic lupus erythematosus. Arthritis Rheum. 1987 May;30(5):513–518. doi: 10.1002/art.1780300505. [DOI] [PubMed] [Google Scholar]
- Mackiewicz A., Pawłowski T., Mackiewicz-Pawłowska A., Wiktorowicz K., Mackiewicz S. Microheterogeneity forms of alpha 1-acid glycoprotein as indicators of rheumatoid arthritis activity. Clin Chim Acta. 1987 Mar 16;163(2):185–190. doi: 10.1016/0009-8981(87)90021-0. [DOI] [PubMed] [Google Scholar]
- Magielska-Zero D., Rokita H., Cieszka K., Kurdowska A., Koj A., Sipe J. D., Gauldie J. Comparison of the acute phase response of cultured Morris hepatoma 7777 cells and of rat hepatocytes. Br J Exp Pathol. 1987 Aug;68(4):485–492. [PMC free article] [PubMed] [Google Scholar]
- May L. T., Ghrayeb J., Santhanam U., Tatter S. B., Sthoeger Z., Helfgott D. C., Chiorazzi N., Grieninger G., Sehgal P. B. Synthesis and secretion of multiple forms of beta 2-interferon/B-cell differentiation factor 2/hepatocyte-stimulating factor by human fibroblasts and monocytes. J Biol Chem. 1988 Jun 5;263(16):7760–7766. [PubMed] [Google Scholar]
- Mega T., Lujan E., Yoshida A. Studies on the oligosaccharide chains of human alpha 1-protease inhibitor. I. Isolation of glycopeptides. J Biol Chem. 1980 May 10;255(9):4053–4056. [PubMed] [Google Scholar]
- Narasimhan S., Freed J. C., Schachter H. The effect of a "bisecting" N-acetylglucosaminyl group on the binding of biantennary, complex oligosaccharides to concanavalin A, Phaseolus vulgaris erythroagglutinin (E-PHA), and Ricinus communis agglutinin (RCA-120) immobilized on agarose. Carbohydr Res. 1986 Jun 1;149(1):65–83. doi: 10.1016/s0008-6215(00)90370-7. [DOI] [PubMed] [Google Scholar]
- Nicollet I., Lebreton J. P., Fontaine M., Hiron M. Evidence for alpha-1-acid glycoprotein populations of different pI values after concanavalin A affinity chromatography. Study of their evolution during inflammation in man. Biochim Biophys Acta. 1981 Apr 28;668(2):235–245. doi: 10.1016/0005-2795(81)90031-3. [DOI] [PubMed] [Google Scholar]
- Perlmutter D. H., Cole F. S., Kilbridge P., Rossing T. H., Colten H. R. Expression of the alpha 1-proteinase inhibitor gene in human monocytes and macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(3):795–799. doi: 10.1073/pnas.82.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes J. Variations in the relative proportions of microheterogeneous forms of plasma glycoproteins in pregnancy and disease. Biomed Pharmacother. 1982 Mar;36(2):77–86. [PubMed] [Google Scholar]
- Serbource-Goguel Seta N., Durand G., Corbic M., Agneray J., Fegar J. Alterations in relative proportions of microheterogenous forms of human alpha 1-acid glycoprotein in liver disease. J Hepatol. 1986;2(2):245–252. doi: 10.1016/s0168-8278(86)80083-6. [DOI] [PubMed] [Google Scholar]
- Vaughan L., Lorier M. A., Carrell R. W. alpha 1-Antitrypsin microheterogeneity. Isolation and physiological significance of isoforms. Biochim Biophys Acta. 1982 Mar 4;701(3):339–345. doi: 10.1016/0167-4838(82)90237-0. [DOI] [PubMed] [Google Scholar]
- Yoshima H., Mizuochi T., Ishii M., Kobata A. Structure of the asparagine-linked sugar chains of alpha-fetoprotein purified from human ascites fluid. Cancer Res. 1980 Nov;40(11):4276–4281. [PubMed] [Google Scholar]