Abstract
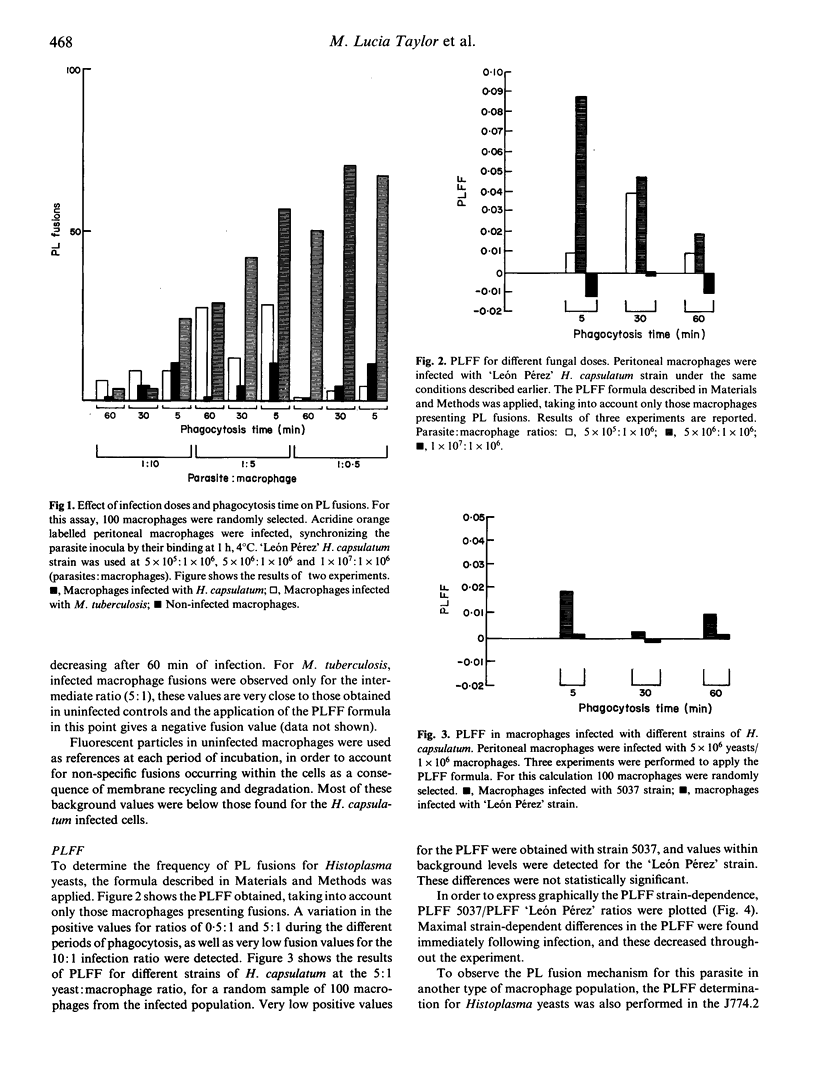
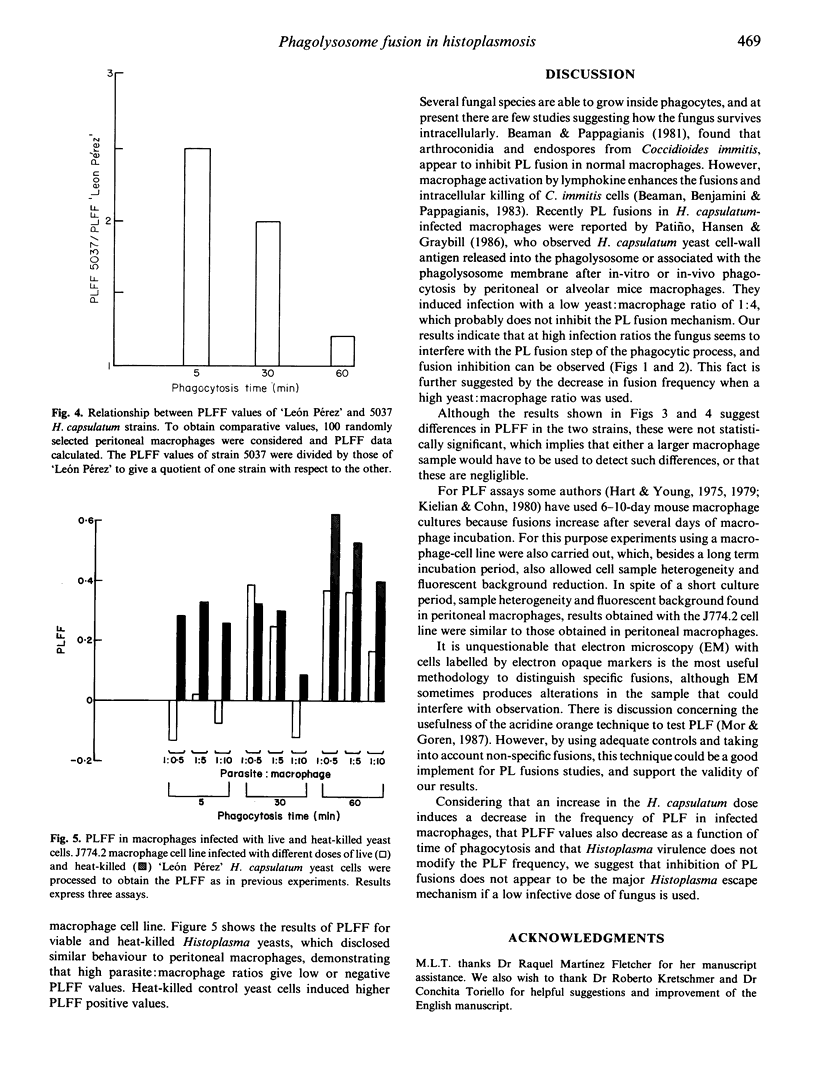
A phagosome-lysosome (PL) fusion was performed in vitro using peritoneal cells from normal BALB/c mice and the J774.2 macrophage cell line infected with the yeast phase of the fungus Histoplasma capsulatum at ratios of 5 x 10(5), 5 x 10(6) or 1 x 10(7) yeasts per 1 x 10(6) macrophages, and phagocytosis was allowed to proceed for 5, 30 and 60 min. Macrophage lysosomes were pre-labelled with acridine orange and the cells were challenged with the parasite. Fusion was evaluated by fluorescence microscopy and the number of macrophages with stained yeast cells was scored. The phagolysosome fusion frequency (PLFF) was calculated by subtracting the specific fusions of infected macrophages from the non-specific fusions of uninfected macrophages and normalizing the total number of bound yeasts. The PLFF was determined using different doses and strains of H. capsulatum. Results showed that PLFF in infected macrophages depends on the infection dose. Inhibition of PL fusion was detected mainly at a high infection ratio (1 x 10(7) yeasts/1 x 10(6) macrophages), and PL fusion varied with phagocytosis time. No significant differences were observed in the fusions when different Histoplasma strains were used. Results with J774.2 cells were similar to peritoneal cells, indicating that both methodology and fusion calculations employed were useful, in spite of the heterogeneity of macrophage populations used.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman L., Benjamini E., Pappagianis D. Activation of macrophages by lymphokines: enhancement of phagosome-lysosome fusion and killing of Coccidioides immitis. Infect Immun. 1983 Mar;39(3):1201–1207. doi: 10.1128/iai.39.3.1201-1207.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Benjamini E., Pappagianis D. Role of lymphocytes in macrophage-induced killing of Coccidioides immitis in vitro. Infect Immun. 1981 Nov;34(2):347–353. doi: 10.1128/iai.34.2.347-353.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Scibienski C., Beaman B. L. Interaction of alveolar macrophages with Nocardia asteroides: immunological enhancement of phagocytosis, phagosome-lysosome fusion, and microbicidal activity. Infect Immun. 1980 Nov;30(2):578–587. doi: 10.1128/iai.30.2.578-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont A., Robert A. Electron microscopic study of phagocytosis of Histoplasma capsulatum by hamster peritoneal macrophages. Lab Invest. 1970 Sep;23(3):278–286. [PubMed] [Google Scholar]
- Frehel C., de Chastellier C., Lang T., Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986 Apr;52(1):252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. The effect of inhibitors and enhancers of phagosome--lysosome fusion in cultured macrophages on the phagosome membranes of ingested yeasts. Exp Cell Res. 1979 Feb;118(2):365–375. doi: 10.1016/0014-4827(79)90160-5. [DOI] [PubMed] [Google Scholar]
- Howard D. H. Fate of Histoplasma capsulatum in guinea pig polymorphonuclear leukocytes. Infect Immun. 1973 Sep;8(3):412–419. doi: 10.1128/iai.8.3.412-419.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H., Otto V. Experiments on lymphocyte-mediated cellular immunity in murine histoplasmosis. Infect Immun. 1977 Apr;16(1):226–231. doi: 10.1128/iai.16.1.226-231.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M. C., Cohn Z. A. Phagosome-lysosome fusion. Characterization of intracellular membrane fusion in mouse macrophages. J Cell Biol. 1980 Jun;85(3):754–765. doi: 10.1083/jcb.85.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor N., Goren M. B. Discrepancy in assessment of phagosome-lysosome fusion with two lysosomal markers in murine macrophages infected with Candida albicans. Infect Immun. 1987 Jul;55(7):1663–1667. doi: 10.1128/iai.55.7.1663-1667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino M. M., Hansen J. T., Graybill J. R. Immunocytochemical staining of Histoplasma capsulatum at the electron microscopic level. Mycopathologia. 1986 Jun;94(3):157–161. doi: 10.1007/BF00454594. [DOI] [PubMed] [Google Scholar]
- Tewari R. P., Sharma D. K., Mathur A. Significance of thymus-derived lymphocytes in immunity elicited by immunization with ribosomes or live yeast cells of Histoplasma capsulatum. J Infect Dis. 1978 Nov;138(5):605–613. doi: 10.1093/infdis/138.5.605. [DOI] [PubMed] [Google Scholar]
- Wu-Hsieh B., Zlotnik A., Howard D. H. T-cell hybridoma-produced lymphokine that activates macrophages to suppress intracellular growth of Histoplasma capsulatum. Infect Immun. 1984 Jan;43(1):380–385. doi: 10.1128/iai.43.1.380-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


