Abstract
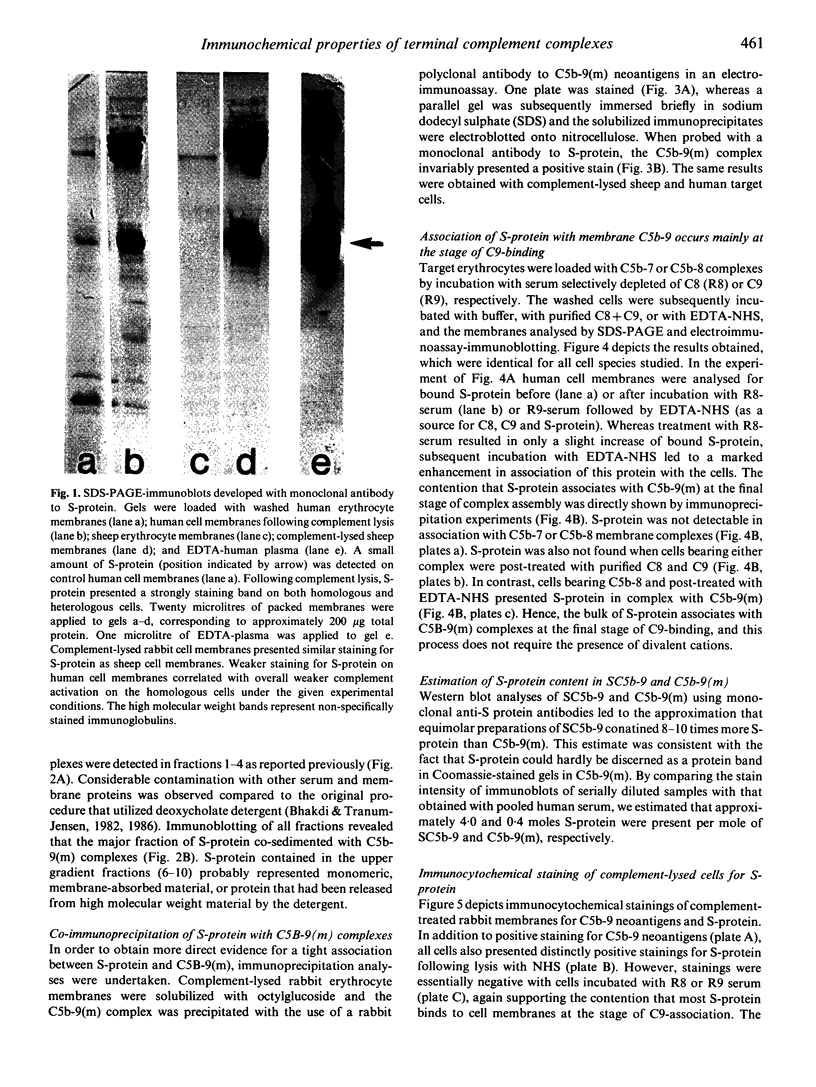
It has been assumed that S-protein (vitronectin) associates with terminal C5b-9 complement complexes only when the latter fail to attach to target lipid bilayers, thereby forming inactive fluid-phase SC5b-9 complexes. Using monoclonal anti-S-protein antibodies, we show here that a minor portion of C5b-9 complexes associated with both homologous and heterologous cells contain S-protein. This conclusion derives from Western blot analyses, from the sedimentation behaviour of solubilized S-protein, and from the fact that the protein co-immunoprecipitates with C5b-9(m). Association of S-protein with C5b-9(m) takes place primarily at the stage of C9-binding. An average of less than or equal to 0.4 moles of S-protein are estimated to be present per mole C5b-9(m). Hence, only a fraction of C5b-9 complexes contain S-protein. The function of cell-bound S-protein is unknown. Haemolytic titrations with purified components failed to demonstrate any protective effect of S-protein on the lysis of sheep or human erythrocytes by C5b-9. S-protein bound to complement-lysed homologous or heterologous cells is readily detectable by conventional immunocytochemical staining. We conclude that differentiation between tissue-deposited fluid-phase C5b-9 and membrane C5b-9 complexes cannot be made on the basis of immunohistological stainings for S-protein alone.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D. W., Silnutzer J. Isolation of human serum spreading factor. J Biol Chem. 1983 Oct 25;258(20):12548–12552. [PubMed] [Google Scholar]
- Barnes D. W., Silnutzer J., See C., Shaffer M. Characterization of human serum spreading factor with monoclonal antibody. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1362–1366. doi: 10.1073/pnas.80.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Ey P., Bhakdi-Lehnen B. Isolation of the terminal complement complex from target sheep erythrocyte membranes. Biochim Biophys Acta. 1976 Feb 6;419(3):445–457. doi: 10.1016/0005-2736(76)90258-3. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Jenne D., Hugo F. Electroimmunoassay-immunoblotting (EIA-IB) for the utilization of monoclonal antibodies in quantitative immunoelectrophoresis: the method and its applications. J Immunol Methods. 1985 Jun 12;80(1):25–32. doi: 10.1016/0022-1759(85)90160-7. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M. A simple immunoradiometric assay for the terminal SC5b-9 complex of human complement. J Immunol Methods. 1983 Feb 25;57(1-3):283–289. doi: 10.1016/0022-1759(83)90088-1. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Roth M. Fluid-phase SC5b-8 complex of human complement: generation and isolation from serum. J Immunol. 1981 Aug;127(2):576–580. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. C5b-9 assembly: average binding of one C9 molecule to C5b-8 without poly-C9 formation generates a stable transmembrane pore. J Immunol. 1986 Apr 15;136(8):2999–3005. [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Membrane damage by complement. Biochim Biophys Acta. 1983 Aug 11;737(3-4):343–372. doi: 10.1016/0304-4157(83)90006-0. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Terminal membrane C5b-9 complex of human complement: transition from an amphiphilic to a hydrophilic state through binding of the S protein from serum. J Cell Biol. 1982 Sep;94(3):755–759. doi: 10.1083/jcb.94.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G. Membrane attack complex of complement as a pathologic mediator. Lab Invest. 1983 Sep;49(3):237–249. [PubMed] [Google Scholar]
- Biesecker G., Müller-Eberhard H. J. The ninth component of human complement: purification and physicochemical characterization. J Immunol. 1980 Mar;124(3):1291–1296. [PubMed] [Google Scholar]
- Couser W. G., Baker P. J., Adler S. Complement and the direct mediation of immune glomerular injury: a new perspective. Kidney Int. 1985 Dec;28(6):879–890. doi: 10.1038/ki.1985.214. [DOI] [PubMed] [Google Scholar]
- Falk R. J., Podack E., Dalmasso A. P., Jennette J. C. Localization of S protein and its relationship to the membrane attack complex of complement in renal tissue. Am J Pathol. 1987 Apr;127(1):182–190. [PMC free article] [PubMed] [Google Scholar]
- Hayman E. G., Engvall E., A'Hearn E., Barnes D., Pierschbacher M., Ruoslahti E. Cell attachment on replicas of SDS polyacrylamide gels reveals two adhesive plasma proteins. J Cell Biol. 1982 Oct;95(1):20–23. doi: 10.1083/jcb.95.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ohgren Y., Ruoslahti E. Serum spreading factor (vitronectin) is present at the cell surface and in tissues. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4003–4007. doi: 10.1073/pnas.80.13.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo F., Jenne D., Bhakdi S. Monoclonal antibodies against neoantigens of the terminal C5b-9 complex of human complement. Biosci Rep. 1985 Aug;5(8):649–658. doi: 10.1007/BF01116996. [DOI] [PubMed] [Google Scholar]
- Hugo F., Krämer S., Bhakdi S. Sensitive ELISA for quantitating the terminal membrane C5b-9 and fluid-phase SC5b-9 complex of human complement. J Immunol Methods. 1987 May 20;99(2):243–251. doi: 10.1016/0022-1759(87)90134-7. [DOI] [PubMed] [Google Scholar]
- Jenne D., Hugo F., Bhakdi S. Monoclonal antibodies to human plasma protein X alias complement S-protein. Biosci Rep. 1985 Apr;5(4):343–352. doi: 10.1007/BF01116907. [DOI] [PubMed] [Google Scholar]
- Jenne D., Stanley K. K. Molecular cloning of S-protein, a link between complement, coagulation and cell-substrate adhesion. EMBO J. 1985 Dec 1;4(12):3153–3157. doi: 10.1002/j.1460-2075.1985.tb04058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb W. P., Muller-Eberhard H. J. The membrane attack mechanism of complement. Isolation and subunit composition of the C5b-9 complex. J Exp Med. 1975 Apr 1;141(4):724–735. [PMC free article] [PubMed] [Google Scholar]
- Mollnes T. E., Harboe M. Immunohistochemical detection of the membrane and fluid-phase terminal complement complexes C5b-9(m) and SC5b-9. Consequences for interpretation and terminology. Scand J Immunol. 1987 Oct;26(4):381–386. doi: 10.1111/j.1365-3083.1987.tb02270.x. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. The membrane attack complex of complement. Annu Rev Immunol. 1986;4:503–528. doi: 10.1146/annurev.iy.04.040186.002443. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Müller-Eberhard H. J. Binding of desoxycholate, phosphatidylcholine vesicles, lipoprotein and of the S-protein to complexes of terminal complement components. J Immunol. 1978 Sep;121(3):1025–1030. [PubMed] [Google Scholar]
- Preissner K. T., Müller-Berghaus G. S protein modulates the heparin-catalyzed inhibition of thrombin by antithrombin III. Evidence for a direct interaction of S protein with heparin. Eur J Biochem. 1986 May 2;156(3):645–650. doi: 10.1111/j.1432-1033.1986.tb09626.x. [DOI] [PubMed] [Google Scholar]
- Preissner K. T., Wassmuth R., Müller-Berghaus G. Physicochemical characterization of human S-protein and its function in the blood coagulation system. Biochem J. 1985 Oct 15;231(2):349–355. doi: 10.1042/bj2310349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer H., Mathey D., Hugo F., Bhakdi S. Deposition of the terminal C5b-9 complement complex in infarcted areas of human myocardium. J Immunol. 1986 Sep 15;137(6):1945–1949. [PubMed] [Google Scholar]
- Shaffer M. C., Foley T. P., Barnes D. W. Quantitation of spreading factor in human biologic fluids. J Lab Clin Med. 1984 May;103(5):783–791. [PubMed] [Google Scholar]
- Steckel E. W., York R. G., Monahan J. B., Sodetz J. M. The eighth component of human complement. Purification and physicochemical characterization of its unusual subunit structure. J Biol Chem. 1980 Dec 25;255(24):11997–12005. [PubMed] [Google Scholar]
- Tschopp J., Masson D., Schäfer S., Peitsch M., Preissner K. T. The heparin binding domain of S-protein/vitronectin binds to complement components C7, C8, and C9 and perforin from cytolytic T-cells and inhibits their lytic activities. Biochemistry. 1988 May 31;27(11):4103–4109. doi: 10.1021/bi00411a029. [DOI] [PubMed] [Google Scholar]







