Abstract
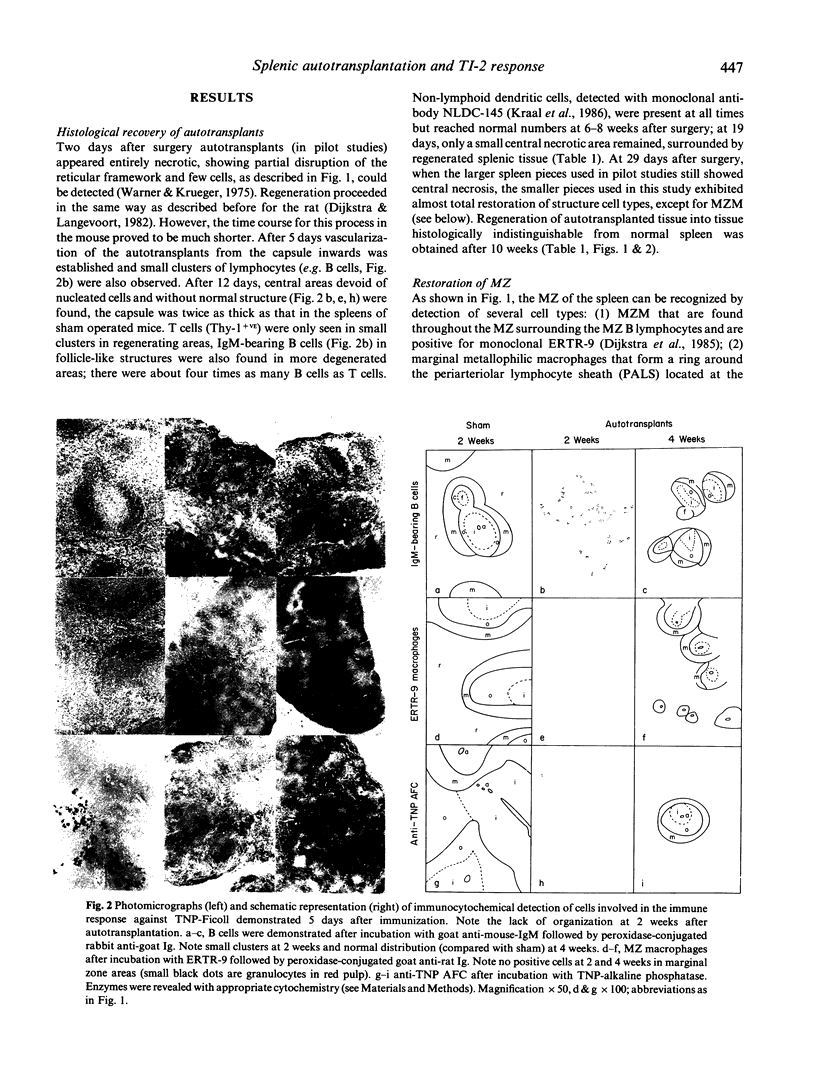
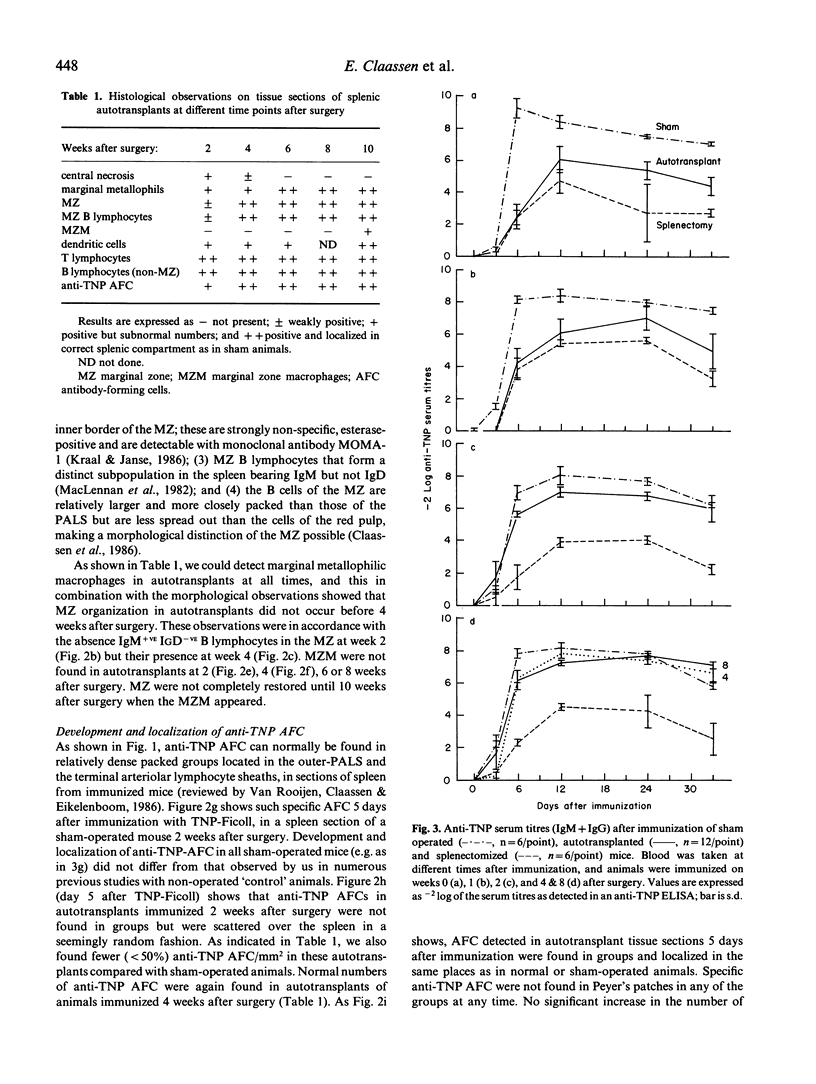
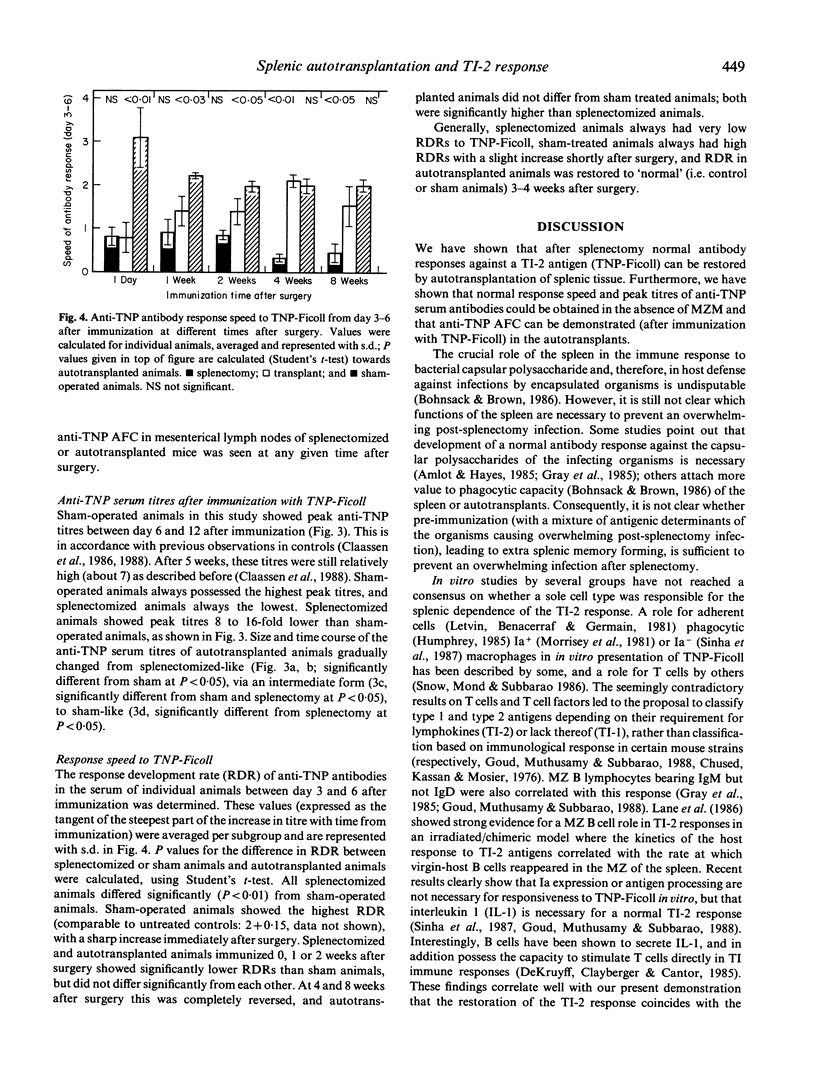
Splenic tissue from mice was autotransplanted; after initial necrosis, a rapid restoration of implants into a structure histologically indistinguishable from splenic tissue was observed. The development of the marginal zone in these autotransplants, as determined with monoclonal antibodies against different splenic cell types and routine histological stains, was compared with the local and systemic response against a thymus-independent (TI) type 2 antigen. Full restoration of time course and peak of anti-trinitrophenyl (TNP) serum titres against TNP-Ficoll was observed at 4 weeks after autotransplantation. Anti-TNP antibody-forming cells were observed in subnormal and normal numbers in 2- and 4-week old autotransplants, respectively. The appearance of normal numbers of antibody-forming cells, and the restoration of antibody titres at week 4 correlated with the return of newly formed B cells in a normal marginal zone. An unexpected observation was that marginal zone macrophages did not return until 10 weeks after transplantation, thereby making the necessity for these cells in the normal TI-2 response unlikely. We conclude that normal anti-TI-2 responses (onset and peak titres) can be restored by autotransplantation of splenic tissue. B cells and marginal zone organization are responsible for this response, for which marginal zone macrophages seem expendable. The partial protection against overwhelming post-splenectomy infections, given by autotransplants, can thus be explained by restorative capabilities of these implants on antigen presentation and antibody formation against TI-2 antigens, and not by an increase (compared with splenectomized individuals) of phagocytosis by marginal zone macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amlot P. L., Hayes A. E. Impaired human antibody response to the thymus-independent antigen, DNP-Ficoll, after splenectomy. Implications for post-splenectomy infections. Lancet. 1985 May 4;1(8436):1008–1011. doi: 10.1016/s0140-6736(85)91613-7. [DOI] [PubMed] [Google Scholar]
- Austrian R., Douglas R. M., Schiffman G., Coetzee A. M., Koornhof H. J., Hayden-Smith S., Reid R. D. Prevention of pneumococcal pneumonia by vaccination. Trans Assoc Am Physicians. 1976;89:184–194. [PubMed] [Google Scholar]
- Bohnsack J. F., Brown E. J. The role of the spleen in resistance to infection. Annu Rev Med. 1986;37:49–59. doi: 10.1146/annurev.me.37.020186.000405. [DOI] [PubMed] [Google Scholar]
- Chused T. M., Kassan S. S., Mosier D. E. Macrophage requirement for the in vitro response to TNP Ficoll: a thymic independent antigen. J Immunol. 1976 Jun;116(6):1579–1581. [PubMed] [Google Scholar]
- Claassen E., Kors N., Dijkstra C. D., Van Rooijen N. Marginal zone of the spleen and the development and localization of specific antibody-forming cells against thymus-dependent and thymus-independent type-2 antigens. Immunology. 1986 Mar;57(3):399–403. [PMC free article] [PubMed] [Google Scholar]
- Claassen E., Kors N., Van Rooijen N. Influence of carriers on the development and localization of anti-2,4,6-trinitrophenyl (TNP) antibody-forming cells in the murine spleen. II. Suppressed antibody response to TNP-Ficoll after elimination of marginal zone cells. Eur J Immunol. 1986 May;16(5):492–497. doi: 10.1002/eji.1830160505. [DOI] [PubMed] [Google Scholar]
- Claassen E., Kors N., van Rooijen N. Immunomodulation with liposomes: the immune response elicited by liposomes with entrapped dichloromethylene-diphosphonate and surface-associated antigen or hapten. Immunology. 1987 Apr;60(4):509–515. [PMC free article] [PubMed] [Google Scholar]
- Claassen E., Westerhof Y., Versluis B., Kors N., Schellekens M., van Rooijen N. Effects of chronic injection of sphingomyelin-containing liposomes on lymphoid and non-lymphoid cells in the spleen. Transient suppression of marginal zone macrophages. Br J Exp Pathol. 1988 Dec;69(6):865–875. [PMC free article] [PubMed] [Google Scholar]
- DeKruyff R. H., Clayberger C., Cantor H. Monoclonal helper T cells induce B cell responses to T-independent antigens: antigen-specific T cells are directly stimulated by activated B cells in the absence of antigen. J Immunol. 1985 Jan;134(1):86–90. [PubMed] [Google Scholar]
- Dijkstra C. D., Langevoort H. L. Regeneration of splenic tissue after autologous subcutaneous implantation: development of non-lymphoid cells in the white pulp of the rat spleen. Cell Tissue Res. 1982;222(1):69–79. doi: 10.1007/BF00218289. [DOI] [PubMed] [Google Scholar]
- Dijkstra C. D., Van Vliet E., Döpp E. A., van der Lelij A. A., Kraal G. Marginal zone macrophages identified by a monoclonal antibody: characterization of immuno- and enzyme-histochemical properties and functional capacities. Immunology. 1985 May;55(1):23–30. [PMC free article] [PubMed] [Google Scholar]
- Goud S. N., Muthusamy N., Subbarao B. Differential responses of B cells from the spleen and lymph node to TNP-Ficoll. J Immunol. 1988 May 1;140(9):2925–2930. [PubMed] [Google Scholar]
- Harding B., Kenny F., Given F., Murphy B., Lavelle S. Autotransplantation of splenic tissue after splenectomy in rats offers partial protection against intravenous pneumococcal challenge. Eur Surg Res. 1987;19(3):135–139. doi: 10.1159/000128692. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H. Splenic macrophages: antigen presenting cells for T1-2 antigens. Immunol Lett. 1985;11(3-4):149–152. doi: 10.1016/0165-2478(85)90161-0. [DOI] [PubMed] [Google Scholar]
- Kraal G., Breel M., Janse M., Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986 Apr 1;163(4):981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986 Aug;58(4):665–669. [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Ter Hart H., Meelhuizen C., Venneker G., Claassen E. Marginal zone macrophages and their role in the immune response against T-independent type 2 antigens: modulation of the cells with specific antibody. Eur J Immunol. 1989 Apr;19(4):675–680. doi: 10.1002/eji.1830190416. [DOI] [PubMed] [Google Scholar]
- Lane P. J., Gray D., Oldfield S., MacLennan I. C. Differences in the recruitment of virgin B cells into antibody responses to thymus-dependent and thymus-independent type-2 antigens. Eur J Immunol. 1986 Dec;16(12):1569–1575. doi: 10.1002/eji.1830161216. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Benacerraf B., Germain R. N. B-lymphocyte responses to trinitrophenyl-conjugated Ficoll: requirement for T lymphocytes and Ia-bearing adherent cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5113–5117. doi: 10.1073/pnas.78.8.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey P. J., Boswell H. S., Scher I., Singer A. Role of accessory cells in B cell activation. IV. Ia+ accessory cells are required for the in vitro generation of thymic independent type 2 antibody responses to polysaccharide antigens. J Immunol. 1981 Oct;127(4):1345–1347. [PubMed] [Google Scholar]
- Rijkers G. T., Mosier D. E. Pneumococcal polysaccharides induce antibody formation by human B lymphocytes in vitro. J Immunol. 1985 Jul;135(1):1–4. [PubMed] [Google Scholar]
- Sinha A. A., Guidos C., Lee K. C., Diener E. Functions of accessory cells in B cell responses to thymus-independent antigens. J Immunol. 1987 Jun 15;138(12):4143–4149. [PubMed] [Google Scholar]
- Snow E. C., Mond J. J., Subbarao B. Enhancement by monoclonal anti-Lyb-2 antibody of antigen-specific B lymphocyte expansion stimulated by TNP-Ficoll and T lymphocyte-derived factors. J Immunol. 1986 Sep 15;137(6):1793–1796. [PubMed] [Google Scholar]
- Warner T. F., Krueger R. G. Regeneration of intact spleen in a heterotropic site in splenectomized mice. Br J Haematol. 1975 Nov;31(3):405–409. doi: 10.1111/j.1365-2141.1975.tb00871.x. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., Kors N., Kraal G. Characterization of cell populations, reappearing in the mouse spleen after elimination by liposome encapsulated dichloromethylene diphosphonate: reappearance of marginal zone lymphocytes is independent of marginal zone macrophages. Adv Exp Med Biol. 1988;237:889–893. doi: 10.1007/978-1-4684-5535-9_133. [DOI] [PubMed] [Google Scholar]