Abstract
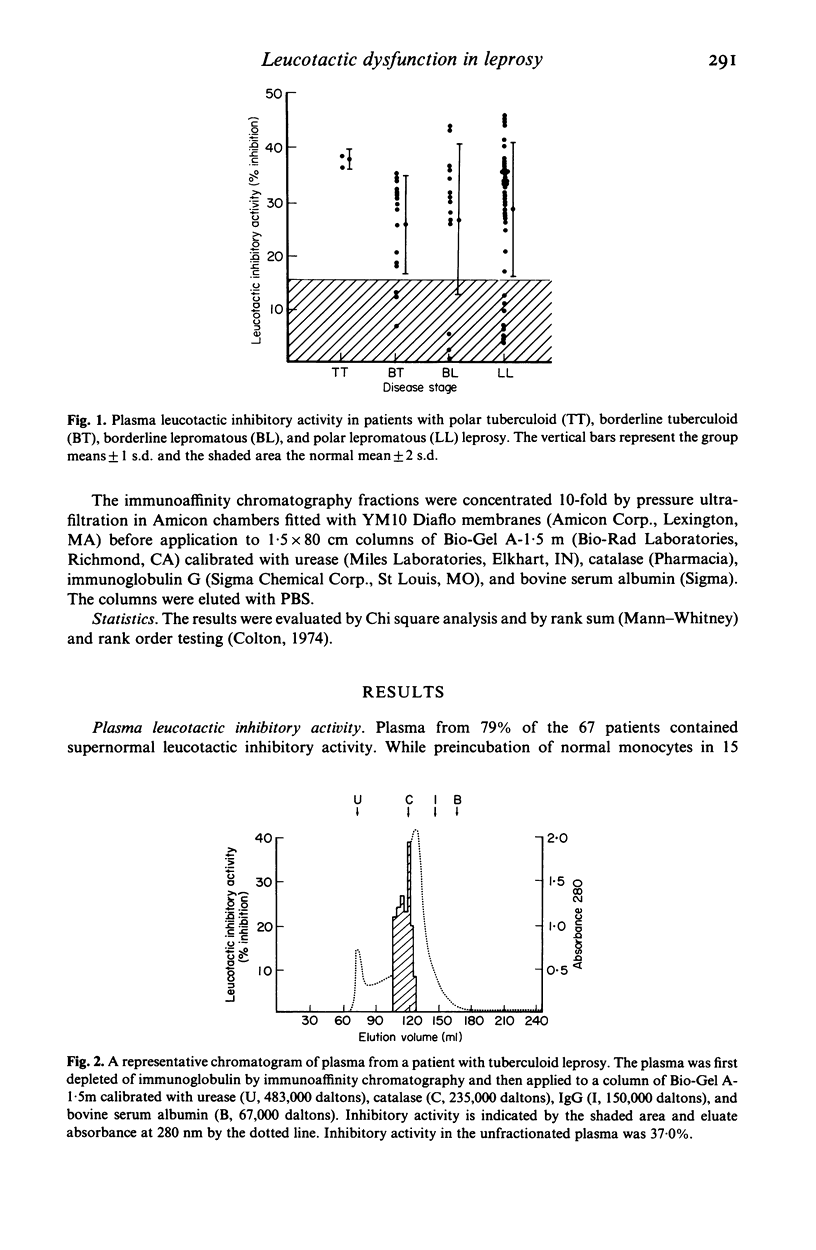
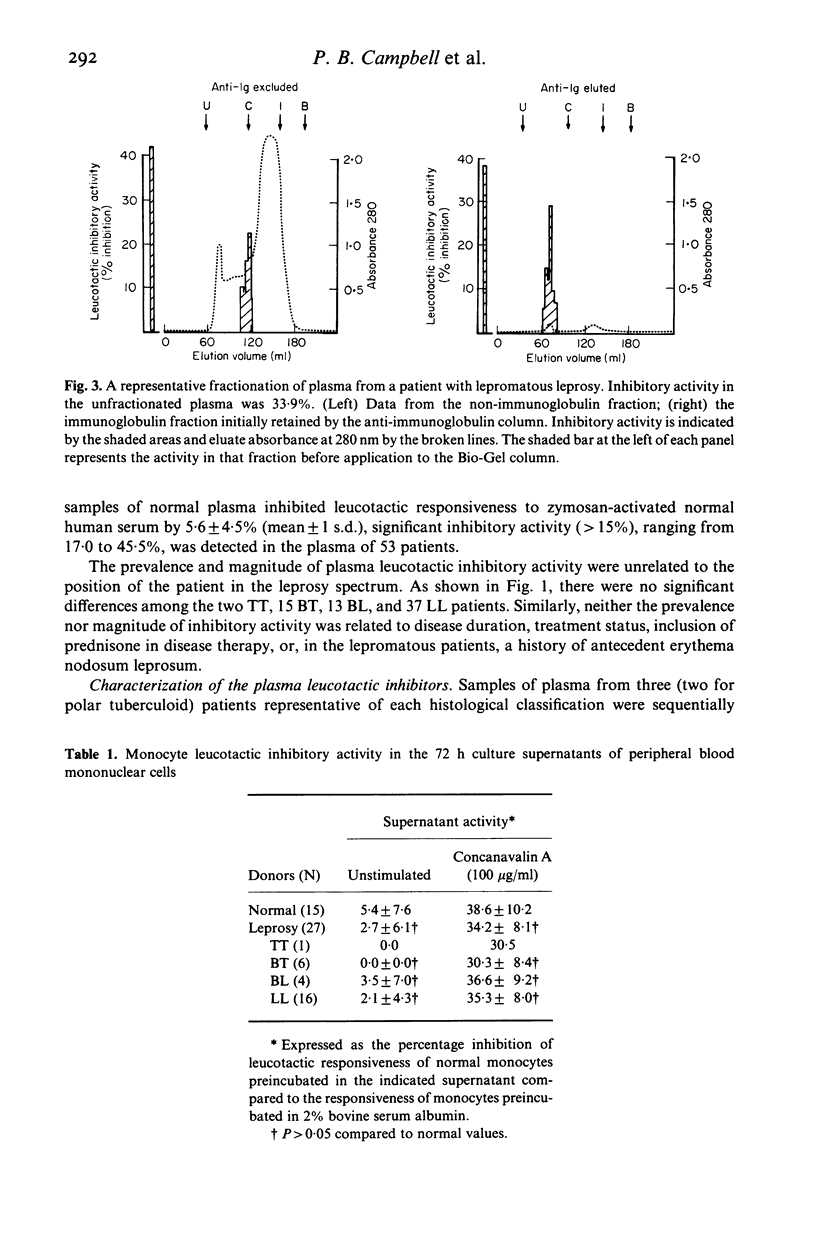
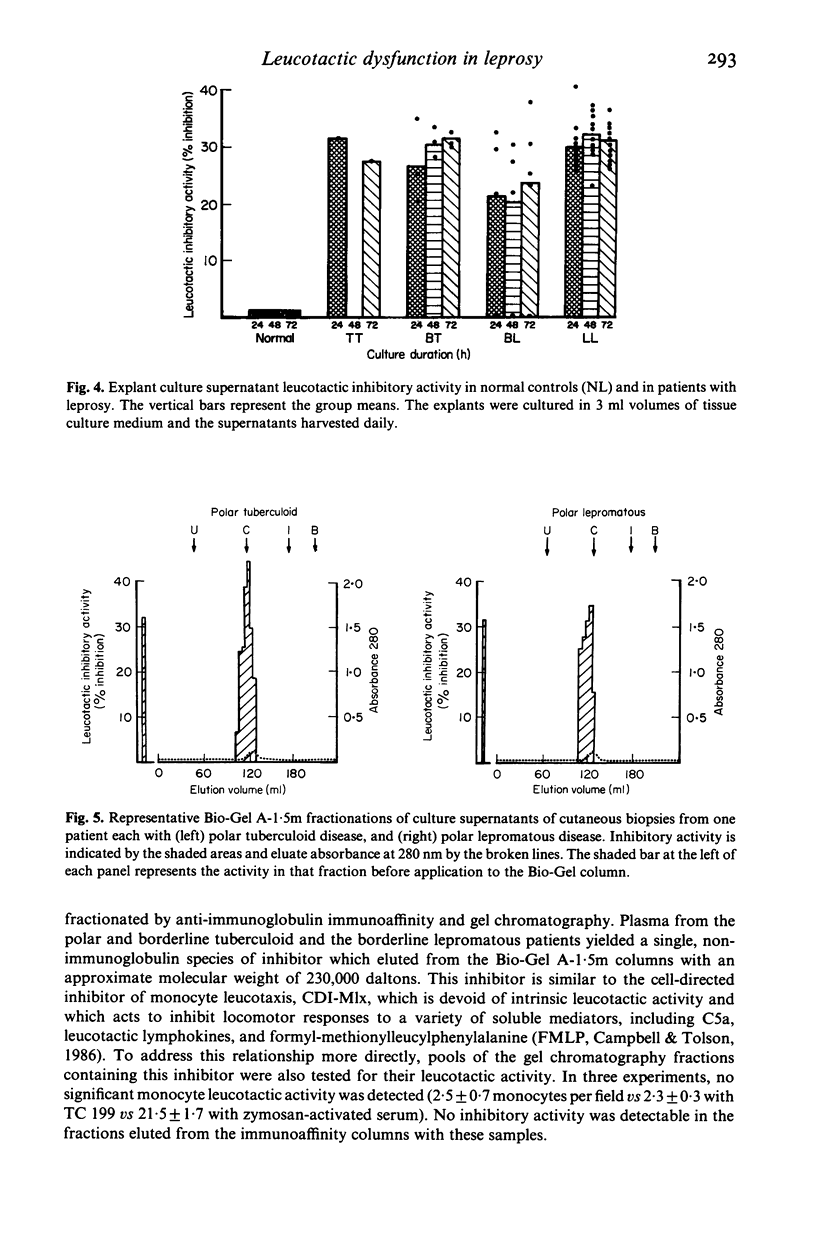
Because the accumulation and activation of mononuclear phagocytes are critical to the host response to intracellular microbial pathogens, we evaluated mechanisms of peripheral monocyte leucotactic regulation in leprosy. Plasma from 53 of 67 patients was found to inhibit the locomotion of normal human monocytes. Neither the prevalence nor the magnitude of plasma leucotactic inhibitory activity correlated with disease histology or duration, type or duration of chemotherapy, or history of erythema nodosum leprosum. Plasma leucotactic inhibitory activity resided principally in a non-immunoglobulin, cell-directed inhibitor of 230,000 daltons molecular weight. Fractionation of plasma from patients with lepromatous leprosy revealed an additional, immunoglobulin-containing inhibitor of approximately 400,000 daltons weight, possibly an IgG-IgA immune complex. Production of leucotactic inhibitors by unstimulated and concanavalin A-stimulated peripheral mononuclear cells was normal; however, cutaneous explants from these patients spontaneously produced the 230,000 dalton leucotactic inhibitor in vitro. The ability of the lesions of leprosy to impede monocyte traffic may be an important pathogenetic mechanism.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai S., Yamamoto H., Itoh K., Kumagai K. Suppressive effect of human natural killer cells on pokeweed mitogen-induced B cell differentiation. J Immunol. 1983 Aug;131(2):651–657. [PubMed] [Google Scholar]
- Azulay R. D. Chemotaxis of monocytes in hanseniasis. Int J Lepr Other Mycobact Dis. 1982 Jun;50(2):215–216. [PubMed] [Google Scholar]
- Baum L. L., James K. K., Glaviano R. R., Gewurz H. Possible role for C-reactive protein in the human natural killer cell response. J Exp Med. 1983 Jan 1;157(1):301–311. doi: 10.1084/jem.157.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill J., Hopper K. E. Immunoregulation by macrophages: differential secretion of prostaglandin E and interleukin 1 during infection with Salmonella enteritidis. Cell Immunol. 1982 Mar 1;67(2):229–240. doi: 10.1016/0008-8749(82)90216-7. [DOI] [PubMed] [Google Scholar]
- Campbell P. B. Defective leukotaxis in monocytes from patients with pulmonary tuberculosis. J Infect Dis. 1979 Apr;139(4):409–417. doi: 10.1093/infdis/139.4.409. [DOI] [PubMed] [Google Scholar]
- Campbell P. B., Thomas J. M., Tolson T. A. A defect of in vitro monocyte leukotaxis induced by cutaneous allograft rejection. Cell Immunol. 1982 Jun;70(1):95–108. doi: 10.1016/0008-8749(82)90136-8. [DOI] [PubMed] [Google Scholar]
- Campbell P. B., Tolson T. A. Natural killer-like cells produce the cell-directed inhibitor of monocyte leukotaxis, CDI-MLx, in vitro. Cell Immunol. 1986 Jan;97(1):67–79. doi: 10.1016/0008-8749(86)90376-x. [DOI] [PubMed] [Google Scholar]
- Djeu J. Y., Stocks N., Zoon K., Stanton G. J., Timonen T., Herberman R. B. Positive self regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes viruses. J Exp Med. 1982 Oct 1;156(4):1222–1234. doi: 10.1084/jem.156.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphres R. C., Gelber R. H., Krahenbuhl J. L. Suppressed natural killer cell activity during episodes of erythema nodosum leprosum in lepromatous leprosy. Clin Exp Immunol. 1982 Sep;49(3):500–508. [PMC free article] [PubMed] [Google Scholar]
- Kasahara T., Djeu J. Y., Dougherty S. F., Oppenheim J. J. Capacity of human large granular lymphocytes (LGL) to produce multiple lymphokines: interleukin 2, interferon, and colony stimulating factor. J Immunol. 1983 Nov;131(5):2379–2385. [PubMed] [Google Scholar]
- Lai A Fat R. F., Chan Pin Jin J., van Furth R., Harboe M. In vitro synthesis of anti-mycobacterial antibodies in biopsies from skin lesions of leprosy patients. Infect Immun. 1980 Feb;27(2):297–301. doi: 10.1128/iai.27.2.297-301.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A Fat R. F., Jin J. C., Diesselhoff-den Dulk M., van Furth R. In vitro synthesis of humoral factors (immunoglobulins and complement) in lesional skin of leprosy patients. Infect Immun. 1979 Sep;25(3):891–895. doi: 10.1128/iai.25.3.891-895.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda A., Scheinberg M. A. Peripheral blood monocyte function in leprosy patients. Int J Lepr Other Mycobact Dis. 1980 Sep;48(3):254–259. [PubMed] [Google Scholar]
- Mortensen R. F., Duszkiewicz J. A. Mediation of CRP-dependent phagocytosis through mouse macrophage Fc-receptors. J Immunol. 1977 Nov;119(5):1611–1616. [PubMed] [Google Scholar]
- Nelson D. S., Penrose J. M., Waters M. F., Pearson J. M., Nelson M. Depressive effect of serum from patients with leprosy on mixed lymphocyte reactions. Influence of anti-leprosy treatment. Clin Exp Immunol. 1975 Dec;22(3):385–392. [PMC free article] [PubMed] [Google Scholar]
- Pistoia V., Cozzolino F., Torcia M., Castigli E., Ferrarini M. Production of B cell growth factor by a Leu-7+, OKM1+ non-T cell with the features of large granular lymphocytes (LGL). J Immunol. 1985 May;134(5):3179–3184. [PubMed] [Google Scholar]
- Ramanathan V. D., Parkash O., Ramu G., Parker D., Curtis J., Sengupta U., Turk J. L. Isolation and analysis of circulating immune complexes in leprosy. Clin Immunol Immunopathol. 1984 Sep;32(3):261–268. doi: 10.1016/0090-1229(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Razin E., Bauminger S., Globerson A. Effect of prostaglandins on phagocytosis of sheep erythrocytes by mouse peritoneal macrophages. J Reticuloendothel Soc. 1978 Apr;23(4):237–242. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Scala G., Allavena P., Djeu J. Y., Kasahara T., Ortaldo J. R., Herberman R. B., Oppenheim J. J. Human large granular lymphocytes are potent producers of interleukin-1. Nature. 1984 May 3;309(5963):56–59. doi: 10.1038/309056a0. [DOI] [PubMed] [Google Scholar]
- Scala G., Allavena P., Ortaldo J. R., Herberman R. B., Oppenheim J. J. Subsets of human large granular lymphocytes (LGL) exhibit accessory cell functions. J Immunol. 1985 May;134(5):3049–3055. [PubMed] [Google Scholar]
- Snyder D. S., Beller D. I., Unanue E. R. Prostaglandins modulate macrophage Ia expression. Nature. 1982 Sep 9;299(5879):163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- Tilden A. B., Abo T., Balch C. M. Suppressor cell function of human granular lymphocytes identified by the HNK-1 (Leu 7) monoclonal antibody. J Immunol. 1983 Mar;130(3):1171–1175. [PubMed] [Google Scholar]
- Wahba A., Cohen H., Sheskin J. Neutrophil chemotactic responses in lepromatous leprosy: an in vitro study of 52 patients. Clin Immunol Immunopathol. 1980 Dec;17(4):556–561. doi: 10.1016/0090-1229(80)90151-8. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Goralnick S., Bullock W. E. Defective leukotaxis in patients with lepromatous leprosy. J Lab Clin Med. 1976 Jun;87(6):1025–1032. [PubMed] [Google Scholar]
- Woronick C. L., Malnick J., Maderazo E. G. Cell-directed inhibitor of human leukocyte locomotion. Identification of immunoglobulin G as a cell-directed inhibitor in normal human serum. J Lab Clin Med. 1981 Jul;98(1):58–67. [PubMed] [Google Scholar]


