Abstract
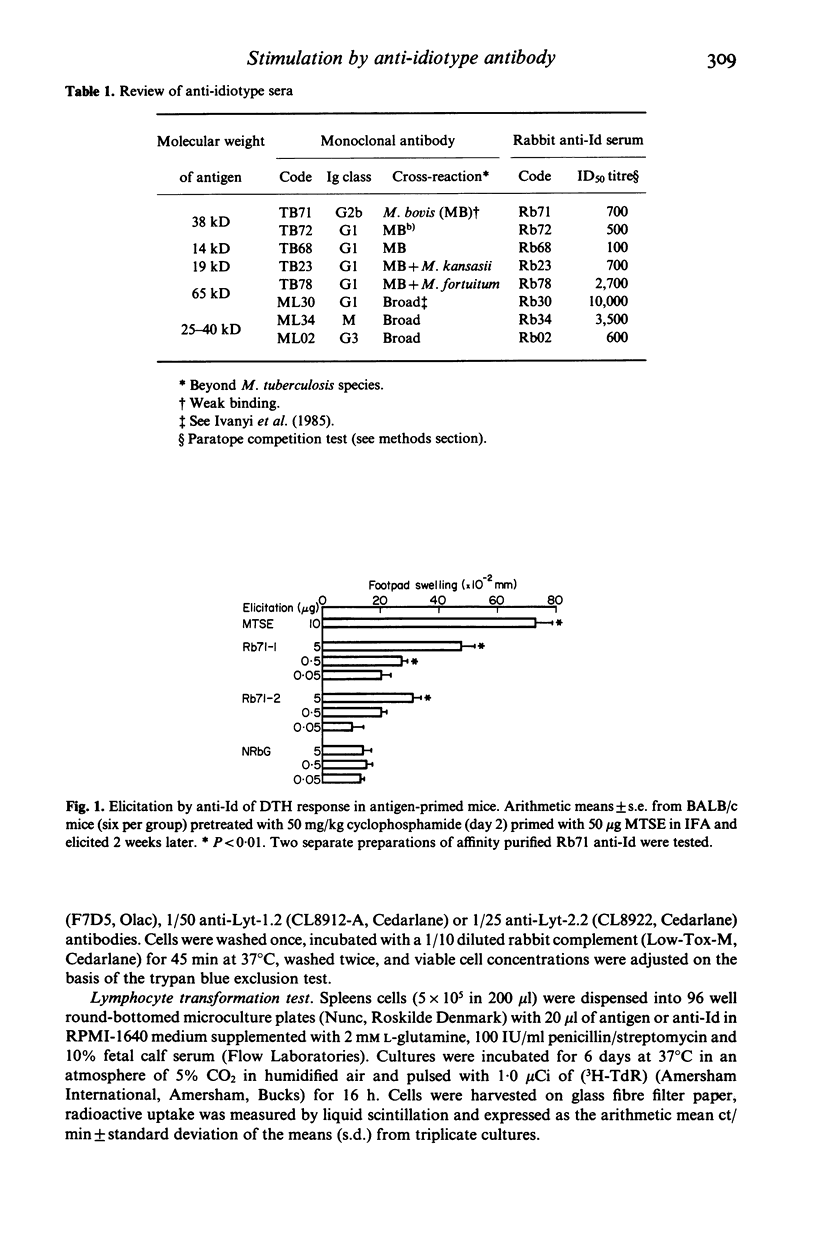
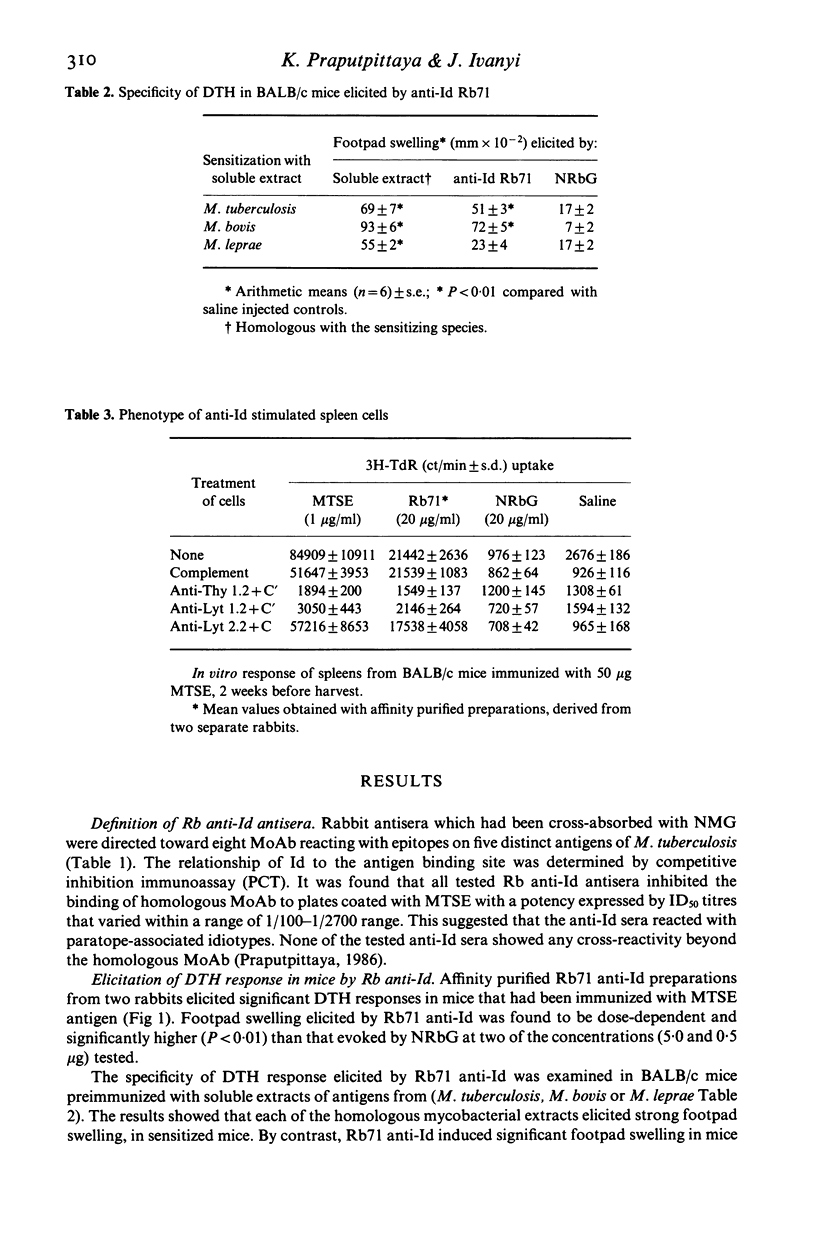
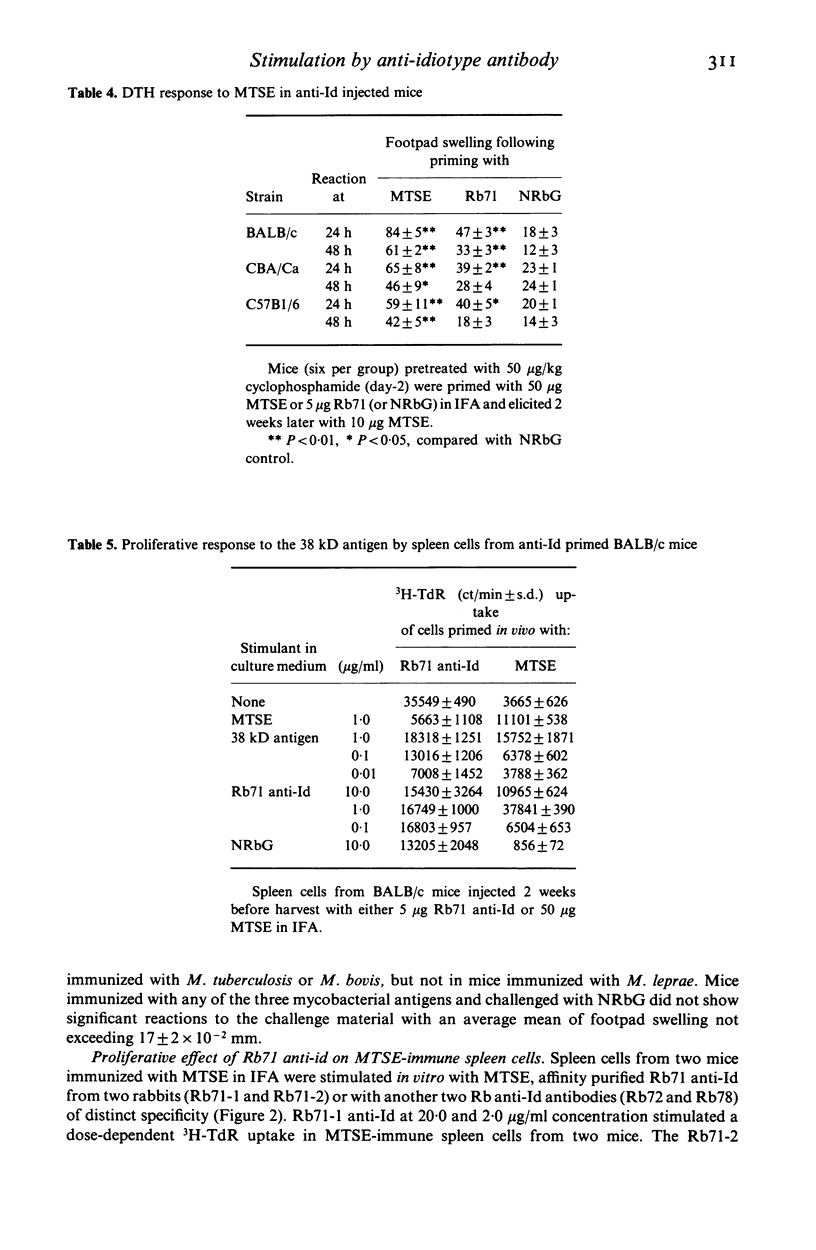
Rabbit antisera raised against eight monoclonal antibodies (MoAb) binding to distinct mycobacterial antigens revealed individual anti-idiotype specificities following cross-absorption with normal mouse globulin. Only one of these antibodies (Rb71), directed towards an M. tuberculosis-specific epitope on the 38 kD protein antigen was able to stimulate mouse T cell responses which had 'internal image' characteristics. This was demonstrated by anti-Id induced in vitro proliferation of Lyt 1.2+ T cells and the elicitation of delayed type hypersensitivity (DTH) foot pad reactions in antigen stimulated BALB/c mice. The DTH reaction was equally strong in mice sensitized with either M. tuberculosis or M. bovis soluble extracts, thus showing a greater degree of cross-reactivity than antibody binding of the corresponding TB71 MoAb. The immunizing potency of the anti-Id in vivo was demonstrable in three strains of mice following injection of Rb71 emulsified with incomplete Freund's adjuvant. These mice showed 38 kD antigen induced DTH-reactions as well as in vitro proliferation of spleen cells. The display of T cell stimulatory internal image by anti-Id may be interpreted as a consequence of binding specificity of TB71 MoAb of a T cell epitope present in the 38 kD antigen.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Binz H., Wigzell H. Shared idiotypic determinants on B and T lymphocytes reactive against the same antigenic determinants. I. Demonstration of similar or identical idiotypes on IgG molecules and T-cell receptors with specificity for the same alloantigens. J Exp Med. 1975 Jul 1;142(1):197–211. doi: 10.1084/jem.142.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Ivanyi J., Sharp K. Control by H-2 genes of murine antibody responses to protein antigens of Mycobacterium tuberculosis. Immunology. 1986 Nov;59(3):329–332. [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. C., Eichberg J. W., Lanford R. E., Dreesman G. R. Anti-idiotypic antibody vaccine for type B viral hepatitis in chimpanzees. Science. 1986 Apr 11;232(4747):220–223. doi: 10.1126/science.3952505. [DOI] [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. Antigen-binding molecules of T cells: distinction from MHC-restricted molecules and segmental homology to immunoglobulin VH and T-cell receptor genes. Scand J Immunol. 1985 Feb;21(2):99–107. doi: 10.1111/j.1365-3083.1985.tb01408.x. [DOI] [PubMed] [Google Scholar]
- McNamara M. K., Ward R. E., Kohler H. Monoclonal idiotope vaccine against Streptococcus pneumoniae infection. Science. 1984 Dec 14;226(4680):1325–1326. doi: 10.1126/science.6505692. [DOI] [PubMed] [Google Scholar]
- Mitsuoka A., Baba M., Morikawa S. Enhancement of delayed hypersensitivity by depletion of suppressor T cells with cyclophosphamide in mice. Nature. 1976 Jul 1;262(5563):77–78. doi: 10.1038/262077a0. [DOI] [PubMed] [Google Scholar]
- Praputpittaya K., Ivanyi J. Study of idiotypes expressed by monoclonal antibodies to the 35 kD and 12 kD antigens of Mycobacterium leprae. Clin Exp Immunol. 1987 Nov;70(2):298–306. [PMC free article] [PubMed] [Google Scholar]
- Rajewsky K., Eichmann K. Antigen receptors of T helper cells. Contemp Top Immunobiol. 1977;7:69–112. doi: 10.1007/978-1-4684-3054-7_3. [DOI] [PubMed] [Google Scholar]
- Ramseier H., Aguet M., Lindenmann J. Similarity of idiotypic determinants of T-and B-lymphocyte receptors for alloantigens. Immunol Rev. 1977;34:50–88. doi: 10.1111/j.1600-065x.1977.tb00368.x. [DOI] [PubMed] [Google Scholar]
- Rees A. D., Praputpittaya K., Scoging A., Dobson N., Ivanyi J., Young D., Lamb J. R. T-cell activation by anti-idiotypic antibody: evidence for the internal image. Immunology. 1987 Mar;60(3):389–393. [PMC free article] [PubMed] [Google Scholar]
- Roth C., Somme G., Gougeon M. L., Theze J. Induction by monoclonal anti-idiotypic antibodies of an anti-poly(Glu60 Ala30 Tyr10) (GAT) immune response in GAT-responder and GAT-nonresponder mice. Scand J Immunol. 1985 Apr;21(4):361–367. doi: 10.1111/j.1365-3083.1985.tb01442.x. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Esser K. M., Sher A. Immunization of mice against African trypanosomiasis using anti-idiotypic antibodies. J Exp Med. 1982 Apr 1;155(4):1108–1119. doi: 10.1084/jem.155.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Gaulton G. N., McDade K. K., Fields B. N., Greene M. I. Syngeneic monoclonal antiidiotype can induce cellular immunity to reovirus. J Exp Med. 1984 Oct 1;160(4):1195–1205. doi: 10.1084/jem.160.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. R., Morahan G., Walker I. D., Miller J. F. Induction of delayed-type hypersensitivity to azobenzenearsonate by a monoclonal anti-idiotype antibody. J Exp Med. 1981 Mar 1;153(3):743–747. doi: 10.1084/jem.153.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Kent L., Rees A., Lamb J., Ivanyi J. Immunological activity of a 38-kilodalton protein purified from Mycobacterium tuberculosis. Infect Immun. 1986 Oct;54(1):177–183. doi: 10.1128/iai.54.1.177-183.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]


