Abstract
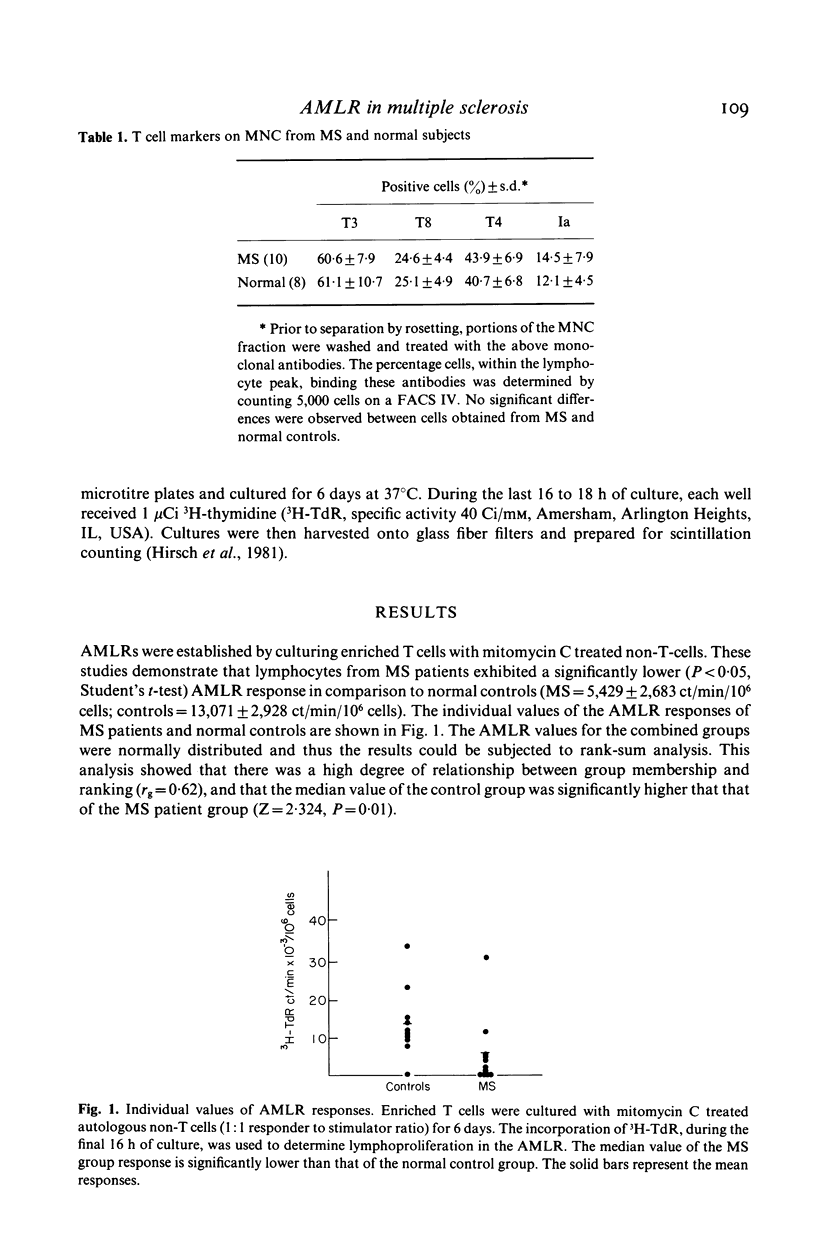
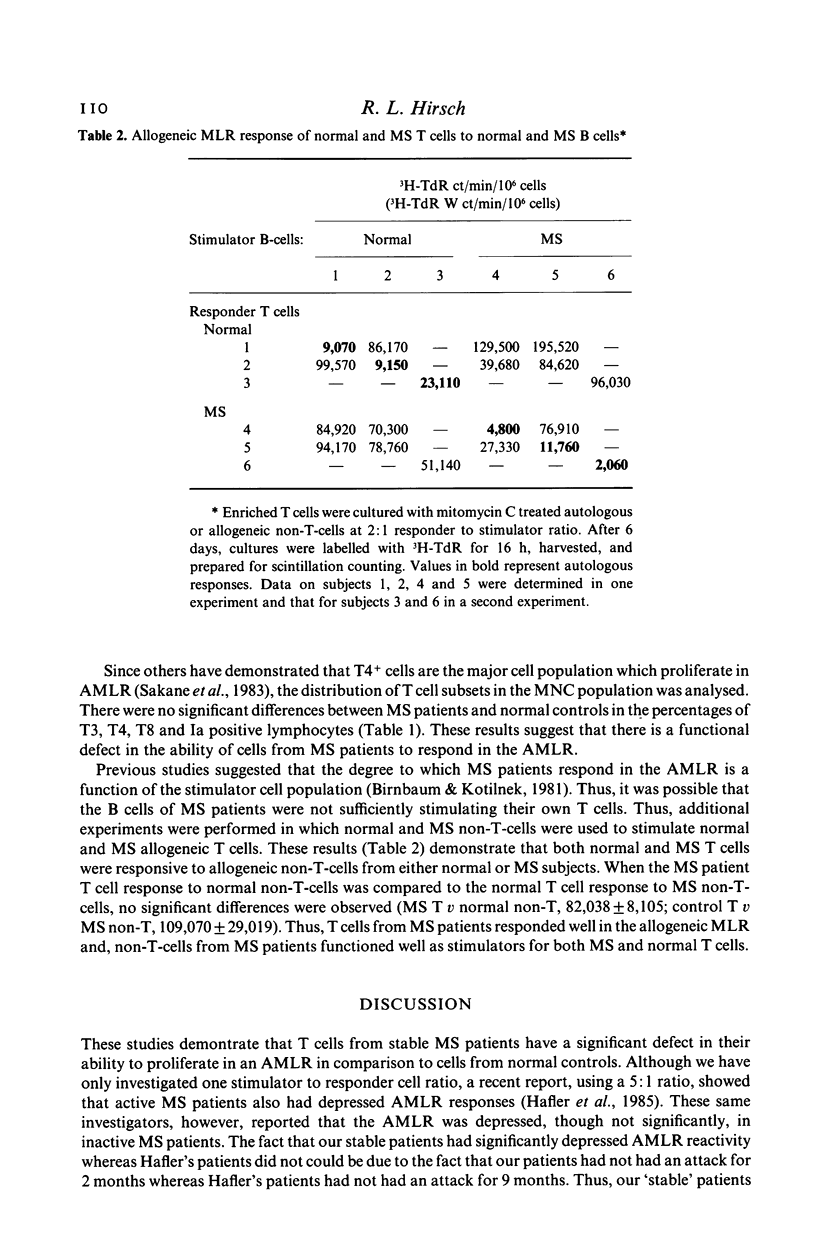
T cells from patients with multiple sclerosis (MS) and normal controls were assessed for their ability to respond in the autologous mixed lymphocyte reaction (AMLR). Cells from stable MS patients demonstrated a significant defect in their proliferative response to non-T cells in comparison to normal controls. Despite the defective AMLR response, T cells from MS patients reacted as well as T cells from normal controls to allogeneic stimuli. Furthermore, MS non-T-cells were fully capable of stimulating allogeneic MLR responses by normal and MS T cells. Since the T4+ cell is the major subpopulation which proliferates in the AMLR, these studies suggest a functional defect in a subpopulation of T4+ cells in MS patients. Since the AMLR may represent an important mechanism by which immune responses are regulated, a defect in the ability of MS T cells to respond to autologous cells could account for several of the autoimmune features of the disease.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzo L. V., Rowley D. A. Homeostasis of the antibody response: immunoregulation by NK cells. Science. 1983 Nov 11;222(4624):581–585. doi: 10.1126/science.6685343. [DOI] [PubMed] [Google Scholar]
- Antel J. P., Arnason B. G., Medof M. E. Suppressor cell function in multiple sclerosis: correlation with clinical disease activity. Ann Neurol. 1979 Apr;5(4):338–342. doi: 10.1002/ana.410050406. [DOI] [PubMed] [Google Scholar]
- Arnason B. G., Antel J. Suppressor cell function in multiple sclerosis. Ann Immunol (Paris) 1978 Feb-Mar;129(2-3):159–170. [PubMed] [Google Scholar]
- Birnbaum G., Kotilinek L. Autologous lymphocyte proliferation in multiple sclerosis and the effect of intravenous ACTH. Ann Neurol. 1981 May;9(5):439–446. doi: 10.1002/ana.410090505. [DOI] [PubMed] [Google Scholar]
- Birnbaum G., Kotilinek L., Schwartz M., Sternad M. Spinal fluid lymphocytes responsive to autologous and allogeneic cells in multiple sclerosis and control individuals. J Clin Invest. 1984 Oct;74(4):1307–1317. doi: 10.1172/JCI111541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieva J. A., Targan S., Stevens R. H. NK and T cell subsets regulate antibody production by human in vivo antigen-induced lymphoblastoid B cells. J Immunol. 1984 Feb;132(2):611–615. [PubMed] [Google Scholar]
- Chandy K. G., Charles A. M., Kershnar A., Buckingham B., Waldeck N., Gupta S. Autologous mixed lymphocyte reaction in man: XV. Cellular and molecular basis of deficient autologous mixed lymphocyte response in insulin-dependent diabetes mellitus. J Clin Immunol. 1984 Nov;4(6):424–428. doi: 10.1007/BF00916571. [DOI] [PubMed] [Google Scholar]
- Coyle P. K., Brooks B. R., Hirsch R. L., Cohen S. R., O'Donnell P., Johnson R. T., Wolinsky J. S. Cerebrospinal-fluid lymphocyte populations and immune complexes in active multiple sclerosis. Lancet. 1980 Aug 2;2(8188):229–232. doi: 10.1016/s0140-6736(80)90121-x. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Gupta S. Autologous mixed lymphocyte reaction in man. V. Functionally and phenotypically distinct human T-cell subpopulations respond to non-T and activated T cells in AMLR. Scand J Immunol. 1982 Jul;16(1):59–68. doi: 10.1111/j.1365-3083.1982.tb00699.x. [DOI] [PubMed] [Google Scholar]
- Goust J. M., Hogan E. L., Arnaud P. Abnormal regulation of IgG production in multiple sclerosis. Neurology. 1982 Mar;32(3):228–234. doi: 10.1212/wnl.32.3.228. [DOI] [PubMed] [Google Scholar]
- Hafler D. A., Buchsbaum M., Weiner H. L. Decreased autologous mixed lymphocyte reaction in multiple sclerosis. J Neuroimmunol. 1985 Oct;9(6):339–347. doi: 10.1016/s0165-5728(85)80034-5. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L., Johnson K. P. The effect of recombinant alpha 2-interferon on defective natural killer cell activity in multiple sclerosis. Neurology. 1985 Apr;35(4):597–600. doi: 10.1212/wnl.35.4.597. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L., Mokhtarian F., Griffin D. E., Brooks B. R., Hess J., Johnson R. T. Measles virus vaccination of measles seropositive individuals suppresses lymphocyte proliferation and chemotactic factor production. Clin Immunol Immunopathol. 1981 Dec;21(3):341–350. doi: 10.1016/0090-1229(81)90223-3. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L., Ordonez J., Panitch H. S., Johnson K. P. T8 antigen density on peripheral blood lymphocytes remains unchanged during exacerbations of multiple sclerosis. J Neuroimmunol. 1985 Oct;9(6):391–398. doi: 10.1016/s0165-5728(85)80038-2. [DOI] [PubMed] [Google Scholar]
- James S. P., Yenokida G. G., Graeff A. S., Elson C. O., Strober W. Immunoregulatory function of T cells activated in the autologous mixed lymphocyte reaction. J Immunol. 1981 Dec;127(6):2605–2609. [PubMed] [Google Scholar]
- Kam-Hansen S., Andersson R., Link H. Cerebrospinal fluid lymphocytes from patients with multiple sclerosis and aseptic meningo-encephalitis respond in mixed lymphocyte culture. J Neuroimmunol. 1983 Aug;5(1):67–81. doi: 10.1016/0165-5728(83)90027-9. [DOI] [PubMed] [Google Scholar]
- Kastrukoff L. F., Paty D. W. A serial study of peripheral blood T lymphocyte subsets in relapsing-remitting multiple sclerosis. Ann Neurol. 1984 Mar;15(3):250–256. doi: 10.1002/ana.410150308. [DOI] [PubMed] [Google Scholar]
- Kotani H., Takada S., Ueda Y., Murakawa Y., Suzuki N., Sakane T. Activation of immune regulatory circuits among OKT4+ cells by autologous mixed lymphocyte reactions. Clin Exp Immunol. 1984 May;56(2):390–398. [PMC free article] [PubMed] [Google Scholar]
- Kuntz M. M., Innes J. B., Weksler M. E. Lymphocyte transformation induced by autologous cells. IV. Human T-lymphocyte proliferation induced by autologous or allogeneic non-T lymphocytes. J Exp Med. 1976 May 1;143(5):1042–1054. doi: 10.1084/jem.143.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källén B., Löw B., Nilsson O. Mixed leukocyte reaction and hl-a specificity at multiple sclerosis. Acta Neurol Scand. 1975 Mar;51(3):184–192. doi: 10.1111/j.1600-0404.1975.tb07599.x. [DOI] [PubMed] [Google Scholar]
- Levitt D., Griffin N. B., Egan M. L. Mitogen-induced plasma cell differentiation in patients with multiple sclerosis. J Immunol. 1980 May;124(5):2117–2121. [PubMed] [Google Scholar]
- Opelz G., Kiuchi M., Takasugi M., Terasaki P. I. Autologous stimulation of human lymphocyte subpopulation. J Exp Med. 1975 Nov 1;142(5):1327–1333. doi: 10.1084/jem.142.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Möller G. HLA-DR antigens render resting T cells sensitive to interleukin-2 and induce production of the growth factor in the autologous mixed lymphocyte reaction. Cell Immunol. 1981 Sep 1;63(1):143–153. doi: 10.1016/0008-8749(81)90035-6. [DOI] [PubMed] [Google Scholar]
- Panitch H. S., Francis G. S. T-lymphocyte subsets in cerebrospinal fluid in multiple sclerosis. N Engl J Med. 1982 Aug 26;307(9):560–561. doi: 10.1056/nejm198208263070921. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Weiner H. L., Hauser S. L., Cohen J. A., Distaso J. A., Schlossman S. F. Loss of suppressor T cells in active multiple sclerosis. Analysis with monoclonal antibodies. N Engl J Med. 1980 Jul 17;303(3):125–129. doi: 10.1056/NEJM198007173030303. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Finney D., Braheny S. L., Knobler R. L., Sipe J. C., Oldstone M. B. Disease activity markers in multiple sclerosis. Another look at suppressor cells defined by monoclonal antibodies OKT4, OKT5, and OKT8. J Neuroimmunol. 1984 Apr;6(2):75–84. doi: 10.1016/0165-5728(84)90028-6. [DOI] [PubMed] [Google Scholar]
- Sakane T., Green I. Specificity and suppressor function of human T cells responsive to autologous non-T cells. J Immunol. 1979 Aug;123(2):584–589. [PubMed] [Google Scholar]
- Sakane T., Kotani H., Takada S., Murakawa Y., Ueda Y. A defect in the suppressor circuits among OKT4+ cell populations in patients with systemic lupus erythematosus occurs independently of a defect in the OKT8+ suppressor T cell function. J Immunol. 1983 Aug;131(2):753–761. [PubMed] [Google Scholar]
- Sandberg-Wollheim M. Lymphocyte populations in the cerebrospinal fluid and peripheral blood of patients with multiple sclerosis and optic neuritis. Scand J Immunol. 1983 Jun;17(6):575–581. doi: 10.1111/j.1365-3083.1983.tb00826.x. [DOI] [PubMed] [Google Scholar]
- Shin H. S., Wang C. Y., Choi Y. S. Activation of autologous reactive helper T lymphocytes for differentiation of human B lymphocytes. J Immunol. 1981 Jun;126(6):2485–2489. [PubMed] [Google Scholar]
- Smolen J. S., Chused T. M., Novotny E. A., Steinberg A. D. The human autologous mixed lymphocyte reaction. III. Immune circuits. J Immunol. 1982 Sep;129(3):1050–1053. [PubMed] [Google Scholar]
- Takada S., Ueda Y., Suzuki N., Murakawa Y., Hoshino T., Green I., Steinberg A. D., Horwitz D. A., Sakane T. Abnormalities in autologous mixed lymphocyte reaction-activated immunologic processes in systemic lupus erythematosus and their possible correction by interleukin 2. Eur J Immunol. 1985 Mar;15(3):262–267. doi: 10.1002/eji.1830150310. [DOI] [PubMed] [Google Scholar]
- Thomas Y., Rogozinski L., Irigoyen O. H., Shen H. H., Talle M. A., Goldstein G., Chess L. Functional analysis of human T cell subsets defined by monoclonal antibodies. V. Suppressor cells within the activated OKT4+ population belong to a distinct subset. J Immunol. 1982 Mar;128(3):1386–1390. [PubMed] [Google Scholar]
- Tomonari K. Cytotoxic T cells generated in the autologous mixed lymphocyte reaction. I. Primary autologous mixed lymphocyte reaction. J Immunol. 1980 Mar;124(3):1111–1121. [PubMed] [Google Scholar]
- Weiner H. L., Hafler D. A., Fallis R. J., Johnson D., Ault K. A., Hauser S. L. Altered blood T-cell subsets in patients with multiple sclerosis. J Neuroimmunol. 1984 Apr;6(2):115–121. doi: 10.1016/0165-5728(84)90032-8. [DOI] [PubMed] [Google Scholar]


