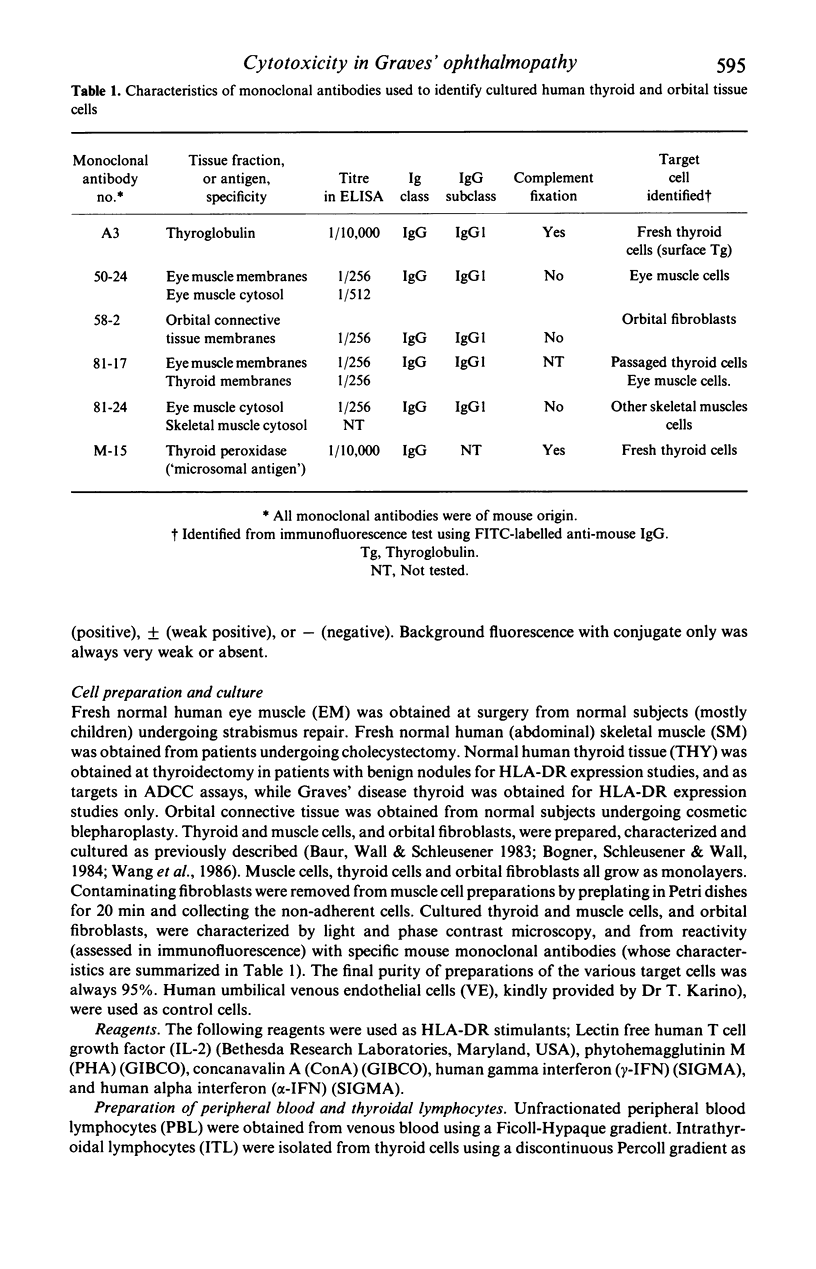
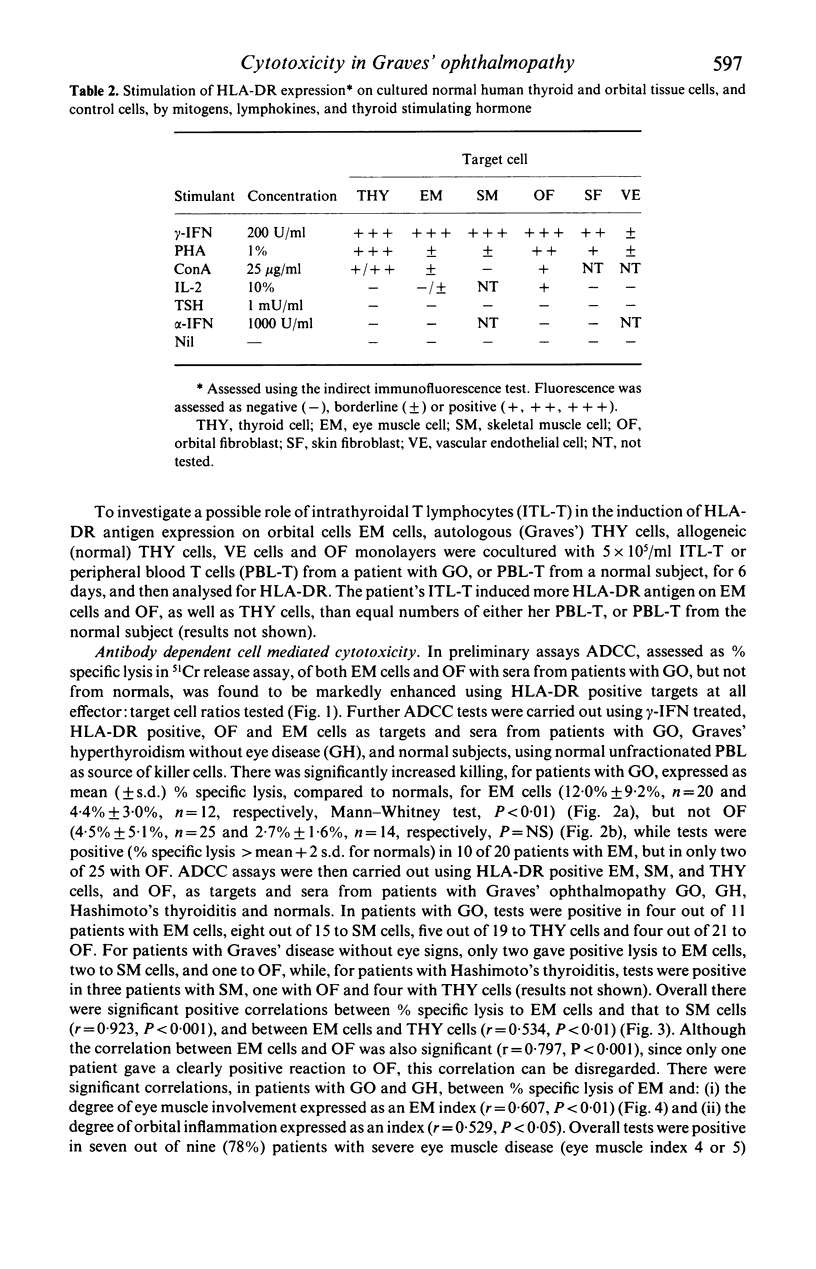
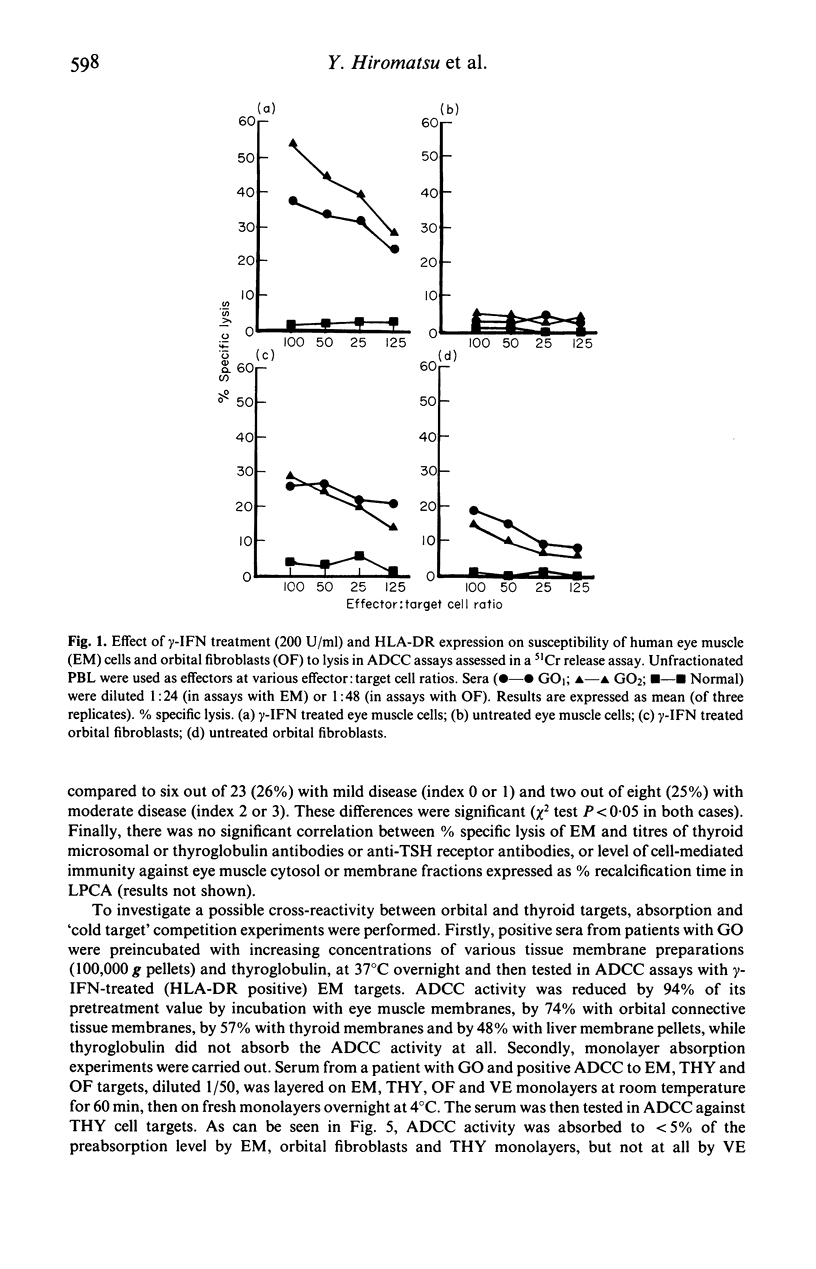
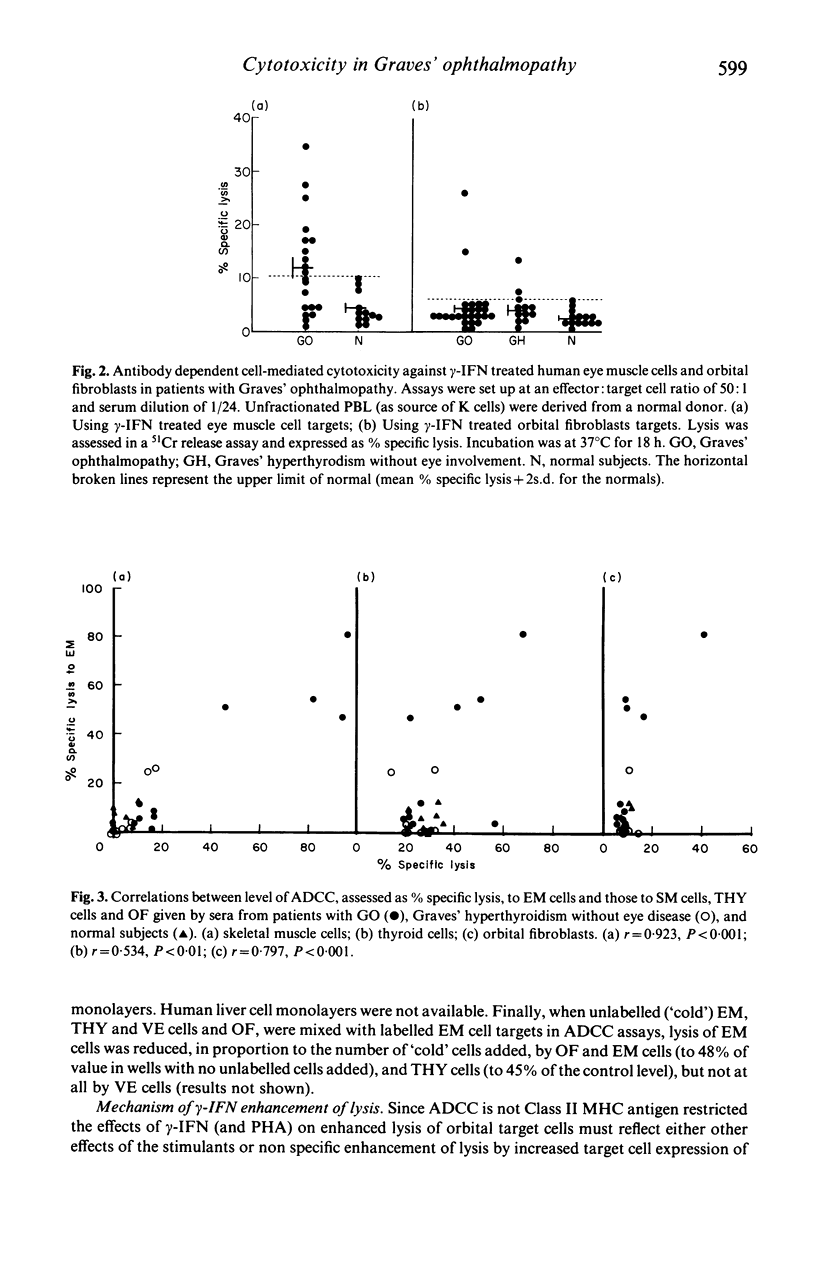
Abstract
We have studied the significance of antibody dependent cell-mediated cytotoxicity (ADCC) against human orbital fibroblasts (OF) and eye muscle (EM) cells in the pathogenesis of the orbital autoimmune reactions of Graves' ophthalmopathy (GO). Possible roles of Class II MHC antigen expression on the surface of orbital target cells and of gamma-interferon (gamma-IFN) modulation of ADCC were also studied. Both OF and EM expressed HLA-DR antigen when stimulated with gamma-IFN and phytohemagglutinin, but not spontaneously, and not by thyroid stimulating hormone or alpha-IFN. Intrathyroidal T cells from a patient with GO induced greater DR expression on both OF and EM cells than equal numbers of her peripheral blood T cells. gamma-IFN treated EM and OF were more susceptible to lysis in ADCC assays than untreated targets. gamma-IFN also enhanced lysis in ADCC assays by an effect on the killer cell population. On the other hand treatment with alpha-IFN, which is a potent inducer of Class I antigen expression, did not affect the susceptibility of target cells to lysis in ADCC. When sera from patients with GO were tested in ADCC, tests were positive (% specific lysis greater than mean + 2 s.d. for normals) in 10 of 20 patients with EM cells, but in only two of 25 with OF. The degree of killing of EM cells was significantly positively correlated to that of abdominal skeletal muscle cells and, to a lesser degree, normal thyroid cells, but not OF. In sera showing killing of EM cells and OF, ADCC activity against EM cells was absorbed by preincubation with EM and orbital connective tissue membranes but not thyroglobulin and, conversely, lysis of THY cells was absorbed by preincubation of positive sera on monolayers of THY and EM cells and OF, but not vascular endothelial (VE) cells. Finally, killing of 51Cr labelled EM cells was inhibited by addition of unlabelled ('cold') thyroid cells, EM cells and OF, but not VE cells. Our findings suggest that ADCC is likely to be an important mechanism for the eye muscle cell damage of GO, but not for the associated orbital connective tissue inflammation. Since ADCC is not MHC-restricted the enhanced lysis of HLA-DR positive target cells presumably reflects other effects of gamma-IFN treatment on both the killer cell population and the target cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amento E. P., Bhan A. K., McCullagh K. G., Krane S. M. Influences of gamma interferon on synovial fibroblast-like cells. Ia induction and inhibition of collagen synthesis. J Clin Invest. 1985 Aug;76(2):837–848. doi: 10.1172/JCI112041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R. J., Wall J. R., Schleusener H. Isolation and characterization of mononuclear cells from various thyroid tissue specimens. Life Sci. 1983 Jan 3;32(1-2):55–65. doi: 10.1016/0024-3205(83)90173-x. [DOI] [PubMed] [Google Scholar]
- Bogner U., Schleusener H., Wall J. R. Antibody-dependent cell mediated cytotoxicity against human thyroid cells in Hashimoto's thyroiditis but not Graves' disease. J Clin Endocrinol Metab. 1984 Oct;59(4):734–738. doi: 10.1210/jcem-59-4-734. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Carrel S., Schmidt-Kessen A., Giuffrè L. Recombinant interferon-gamma can induce the expression of HLA-DR and -DC on DR-negative melanoma cells and enhance the expression of HLA-ABC and tumor-associated antigens. Eur J Immunol. 1985 Feb;15(2):118–123. doi: 10.1002/eji.1830150204. [DOI] [PubMed] [Google Scholar]
- Davies T. F., Piccinini L. A. HLA class II antigens and the human thyroid cell: a review. Mt Sinai J Med. 1986 Jan;53(1):23–30. [PubMed] [Google Scholar]
- Faryna M., Nauman J., Gardas A. Measurement of autoantibodies against human eye muscle plasma membranes in Graves' ophthalmopathy. Br Med J (Clin Res Ed) 1985 Jan 19;290(6463):191–192. doi: 10.1136/bmj.290.6463.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. Ophthalmopathy of Graves' disease. N Engl J Med. 1983 Feb 24;308(8):453–454. doi: 10.1056/NEJM198302243080811. [DOI] [PubMed] [Google Scholar]
- Guyre P. M., Morganelli P. M., Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983 Jul;72(1):393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Jansson R., Karlsson A., Forsum U. Intrathyroidal HLA-DR expression and T lymphocyte phenotypes in Graves' thyrotoxicosis, Hashimoto's thyroiditis and nodular colloid goitre. Clin Exp Immunol. 1984 Nov;58(2):264–272. [PMC free article] [PubMed] [Google Scholar]
- Kodama K., Sikorska H., Bandy-Dafoe P., Bayly R., Wall J. R. Demonstration of a circulating autoantibody against a soluble eye-muscle antigen in Graves' ophthalmopathy. Lancet. 1982 Dec 18;2(8312):1353–1356. doi: 10.1016/s0140-6736(82)91267-3. [DOI] [PubMed] [Google Scholar]
- Kuroki T., Ruf J., Whelan L., Miller A., Wall J. R. Antithyroglobulin monoclonal and autoantibodies cross-react with an orbital connective tissue membrane antigen: a possible mechanism for the association of ophthalmopathy with autoimmune thyroid disorders. Clin Exp Immunol. 1985 Nov;62(2):361–370. [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Dallman M., Barclay A. N. Graft-versus-host disease induces expression of Ia antigen in rat epidermal cells and gut epithelium. Nature. 1981 Sep 10;293(5828):150–151. doi: 10.1038/293150a0. [DOI] [PubMed] [Google Scholar]
- Mengistu M., Laryea E., Miller A., Wall J. R. Clinical significance of a new autoantibody against a human eye muscle soluble antigen, detected by immunofluorescence. Clin Exp Immunol. 1986 Jul;65(1):19–27. [PMC free article] [PubMed] [Google Scholar]
- Munro R. E., Lamki L., Row V. V., Volpé R. Cell-mediated immunity in the exophthalmos of Graves' disease as demonstrated by the migration inhibition factor (MIF) test. J Clin Endocrinol Metab. 1973 Aug;37(2):286–292. doi: 10.1210/jcem-37-2-286. [DOI] [PubMed] [Google Scholar]
- Pujol-Borrell R., Hanafusa T., Chiovato L., Bottazzo G. F. Lectin-induced expression of DR antigen on human cultured follicular thyroid cells. Nature. 1983 Jul 7;304(5921):71–73. doi: 10.1038/304071a0. [DOI] [PubMed] [Google Scholar]
- Rotella C. M., Zonefrati R., Toccafondi R., Valente W. A., Kohn L. D. Ability of monoclonal antibodies to the thyrotropin receptor to increase collagen synthesis in human fibroblasts: an assay which appears to measure exophthalmogenic immunoglobulins in Graves' sera. J Clin Endocrinol Metab. 1986 Feb;62(2):357–367. doi: 10.1210/jcem-62-2-357. [DOI] [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Granato D., Perussia B. Interferon-induced resistance of fibroblasts to cytolysis mediated by natural killer cells: specificity and mechanism. J Immunol. 1981 Jan;126(1):335–340. [PubMed] [Google Scholar]
- Wang P. W., Hiromatsu Y., Laryea E., Wosu L., How J., Wall J. R. Immunologically mediated cytotoxicity against human eye muscle cells in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1986 Aug;63(2):316–322. doi: 10.1210/jcem-63-2-316. [DOI] [PubMed] [Google Scholar]
- Werner S. C. Modification of the classification of the eye changes of Graves' disease: recommendations of the Ad Hoc Committee of the American Thyroid Association. J Clin Endocrinol Metab. 1977 Jan;44(1):203–204. doi: 10.1210/jcem-44-1-203. [DOI] [PubMed] [Google Scholar]


