Abstract
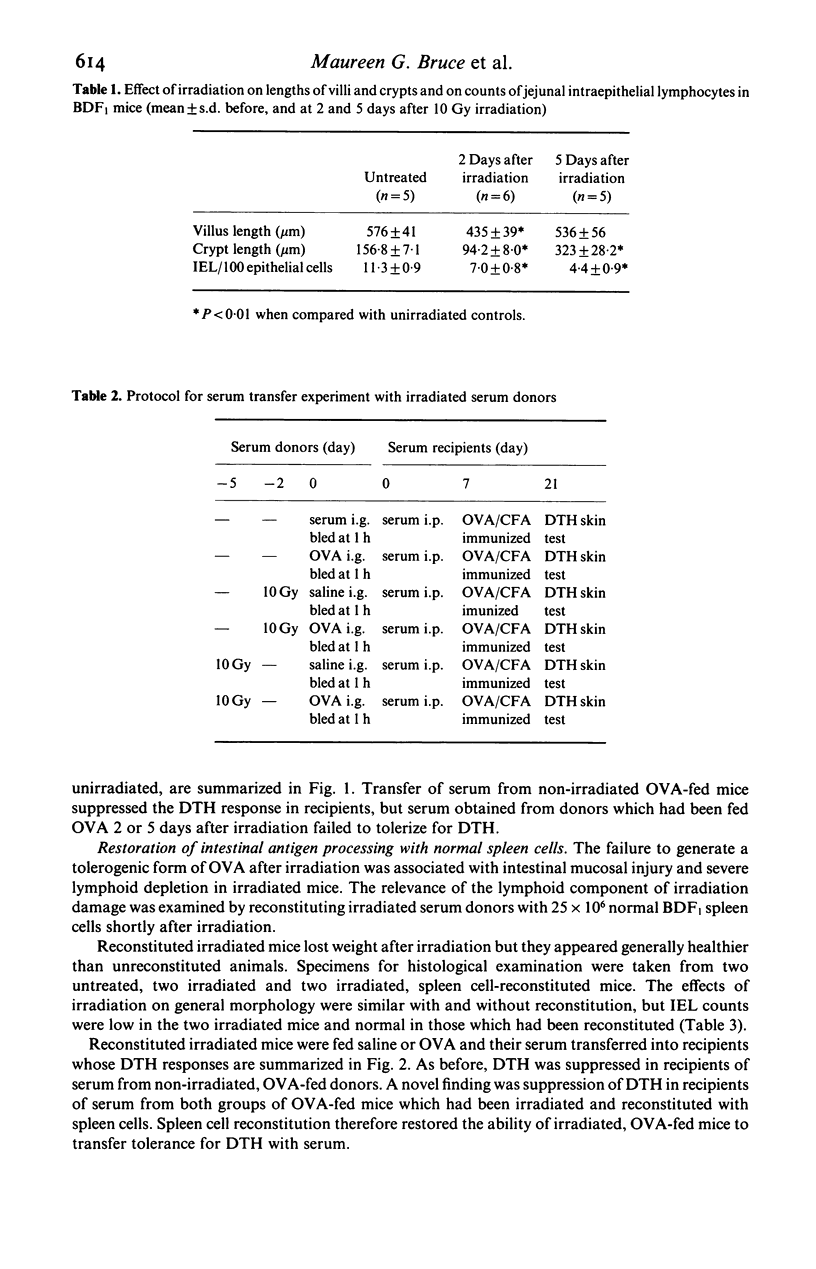
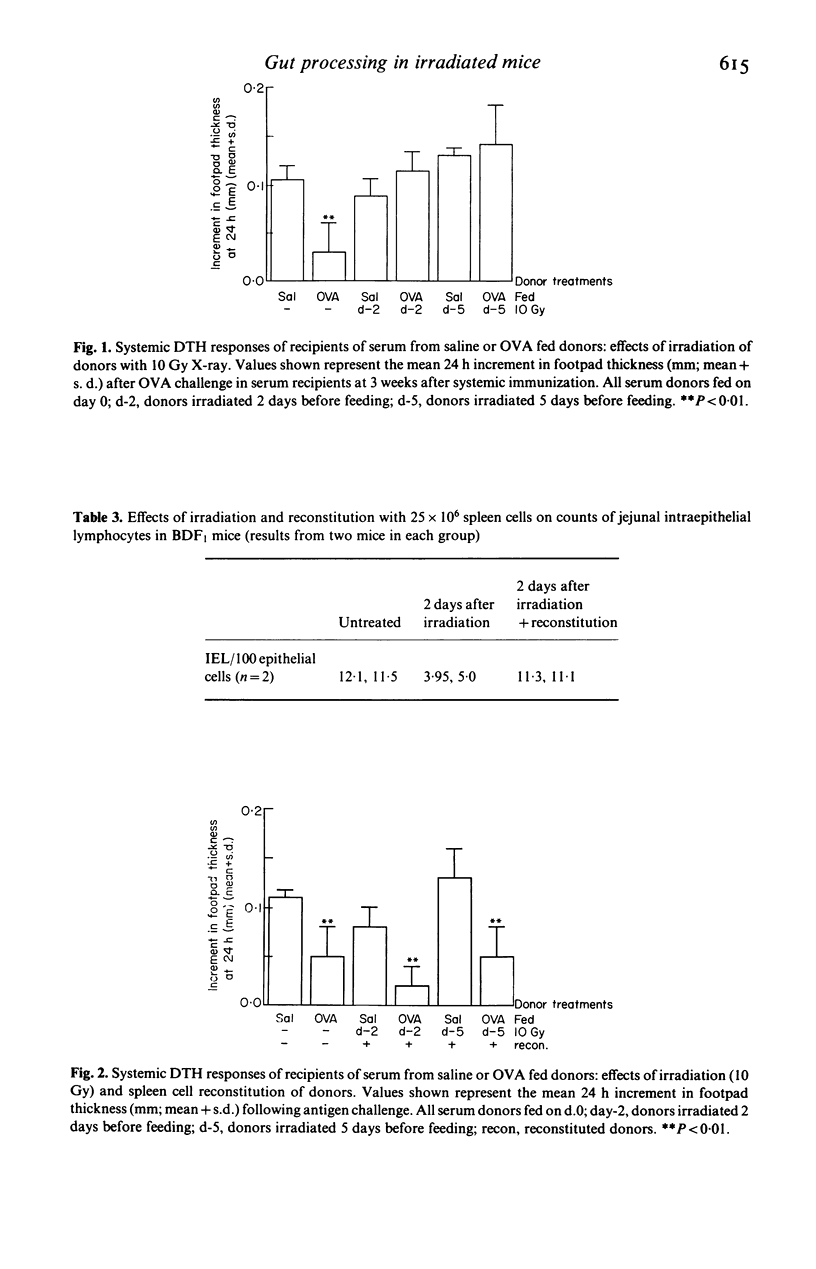
'Intestinal antigen processing' is a function of the gastro-intestinal tract whereby shortly after an animal has been fed an immunogenic protein antigen, such as ovalbumin (OVA), a tolerogenic form of the protein is generated and can be detected in the circulation. The effect of damage to the intestinal epithelium on the processing of OVA has been examined in lethally irradiated mice. Irradiated animals were fed 25 mg OVA and their serum collected 1 h later. When this serum was transferred intraperitoneally into naive recipient mice, this did not induce the typical suppression of systemic delayed-type hypersensitivity. Results were similar when the serum donors were at 2 days after irradiation, with crypt hypoplasia, and at 5 days after irradiation when there was reactive crypt hyperplasia. However reconstitution of donors with normal spleen cells immediately after irradiation restored their capacity to generate a tolerogenic form of the antigen. Immunoreactive OVA was detected by ELISA in both tolerizing and non-tolerizing sera, and the immunological properties of these sera were not related to serum levels of OVA after feeding. Thus subtle immunochemical alterations in the nature of a protein antigen are likely to be more important than the quantity of absorbed antigen, in influencing systemic cell-mediated immune responses after feeding. The lack of generation of a tolerogenic form of the protein in irradiated mice, unrelated to the pattern of epithelial cell kinetics, and the restoration of this function by normal spleen cells, suggests that lymphoid cells may be involved in the phenomenon of antigen processing.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. E., Warner N. L. Ionizing radiation and the immune response. Adv Immunol. 1976;24:215–335. doi: 10.1016/s0065-2776(08)60331-4. [DOI] [PubMed] [Google Scholar]
- Bruce M. G., Ferguson A. Oral tolerance to ovalbumin in mice: studies of chemically modified and 'biologically filtered' antigen. Immunology. 1986 Apr;57(4):627–630. [PMC free article] [PubMed] [Google Scholar]
- Bruce M. G., Ferguson A. The influence of intestinal processing on the immunogenicity and molecular size of absorbed, circulating ovalbumin in mice. Immunology. 1986 Oct;59(2):295–300. [PMC free article] [PubMed] [Google Scholar]
- DETRICK L. E., LATTA H., UPHAM H. C., McCANDLESS R. Electron-microscopic changes across irradiated rat intestinal villi. Radiat Res. 1963 Jul;19:447–461. [PubMed] [Google Scholar]
- Ferguson A., Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971 Dec;12(12):988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson A., Sutherland A., MacDonald T. T., Allan F. Technique for microdissection and measurement in biopsies of human small intestine. J Clin Pathol. 1977 Nov;30(11):1068–1073. doi: 10.1136/jcp.30.11.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaoka T., Katz D. H., Benacerraf B. Radioresistance of carrier-specific helper thymus-derived lymphocytes in mice. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3453–3458. doi: 10.1073/pnas.69.11.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D. G., Vaz N. M., Rawlings L. A., Lynch J. M. Inhibition of specific immune responses by feeding protein antigens. II. Effects of prior passive and active immunization. J Immunol. 1979 Jun;122(6):2261–2266. [PubMed] [Google Scholar]
- Miller S. D., Hanson D. G. Inhibition of specific immune responses by feeding protein antigens. IV. Evidence for tolerance and specific active suppression of cell-mediated immune responses to ovalbumin. J Immunol. 1979 Nov;123(5):2344–2350. [PubMed] [Google Scholar]
- Mowat A. M., Strobel S., Drummond H. E., Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982 Jan;45(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- QUASTLER H., HAMPTON J. C. Effects of ionizing radiation on the fine structure and function of the intestinal epithelium of the mouse. I. Villus epithelium. Radiat Res. 1962 Dec;17:914–931. [PubMed] [Google Scholar]
- Strobel S., Ferguson A. Immune responses to fed protein antigens in mice. 3. Systemic tolerance or priming is related to age at which antigen is first encountered. Pediatr Res. 1984 Jul;18(7):588–594. doi: 10.1203/00006450-198407000-00004. [DOI] [PubMed] [Google Scholar]
- Strobel S., Mowat A. M., Drummond H. E., Pickering M. G., Ferguson A. Immunological responses to fed protein antigens in mice. II. Oral tolerance for CMI is due to activation of cyclophosphamide-sensitive cells by gut-processed antigen. Immunology. 1983 Jul;49(3):451–456. [PMC free article] [PubMed] [Google Scholar]
- Wicker L. S., Katz M., Sercarz E. E., Miller A. Immunodominant protein epitopes. I. Induction of suppression to hen egg white lysozyme is obliterated by removal of the first three N-terminal amino acids. Eur J Immunol. 1984 May;14(5):442–447. doi: 10.1002/eji.1830140511. [DOI] [PubMed] [Google Scholar]


