Abstract
During blood-stage infection of mice with a lethal variant of Plasmodium yoelii, cells in both spleen and liver became activated to reach a peak at day 5. In mice protected by vaccination, activation was accelerated after infection. The most striking difference observed was in the 10-fold greater yield of infiltrating cells, including macrophages, obtained from the liver just before the mice recovered. Their capacity to give an oxidative burst and their cytotoxic activity against tumour cells was also more than 10 times normal. This suggests that the recruitment of inflammatory cells to the liver plays an important role in the protection of vaccinated mice against malaria.
Full text
PDF

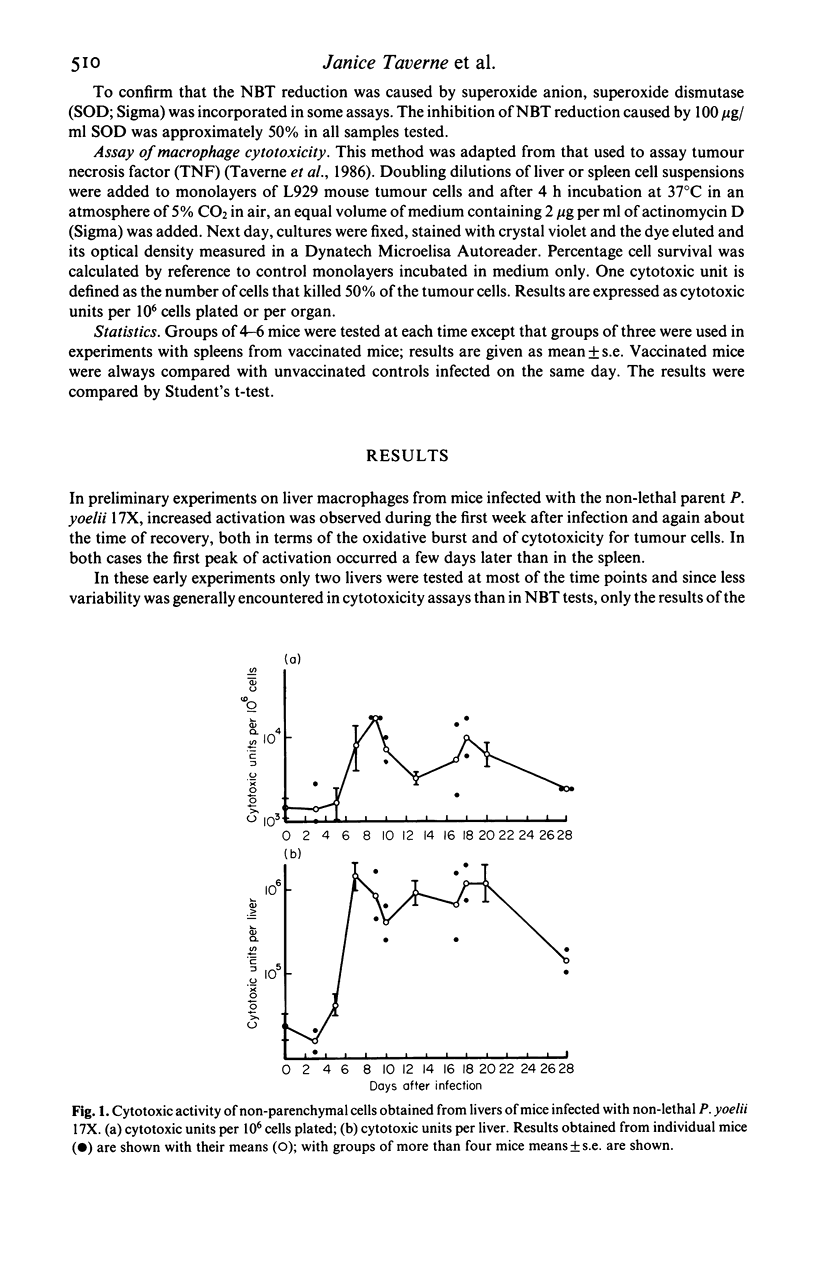
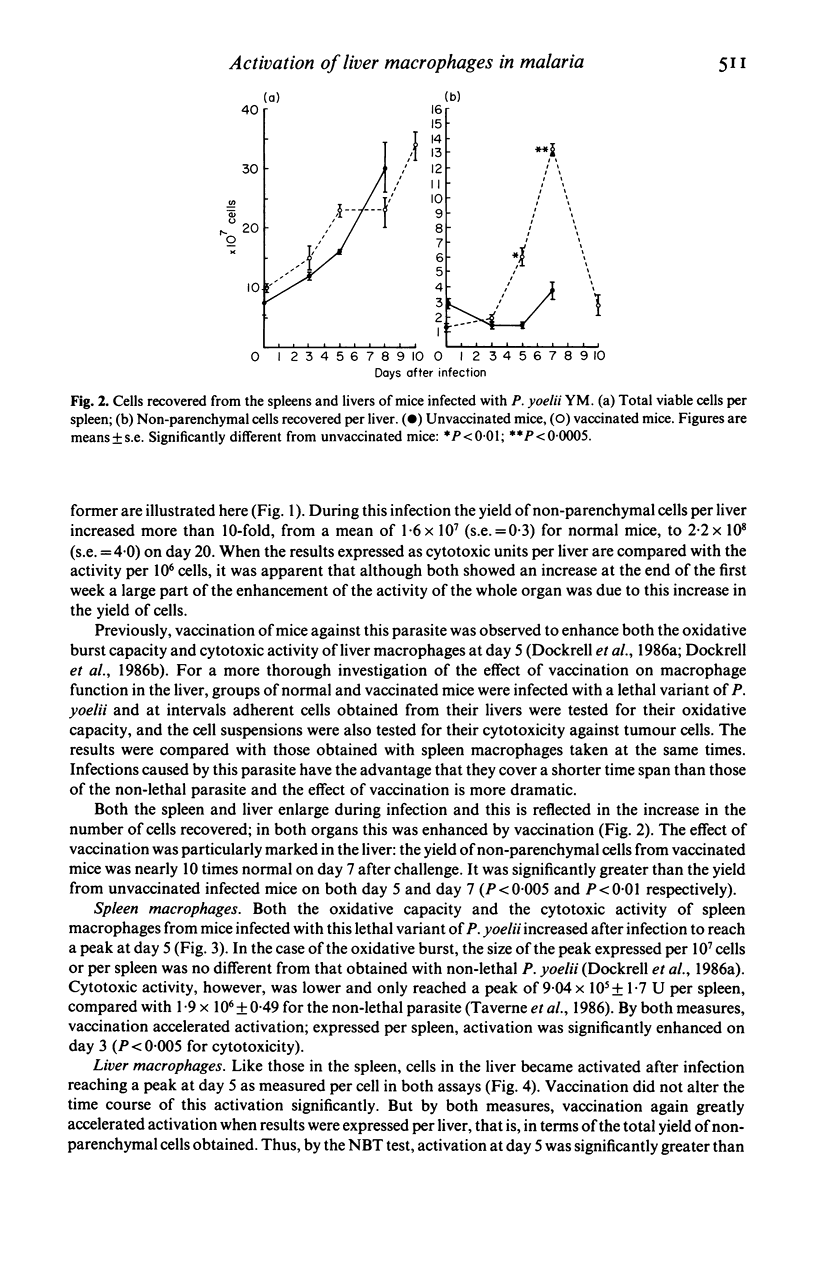
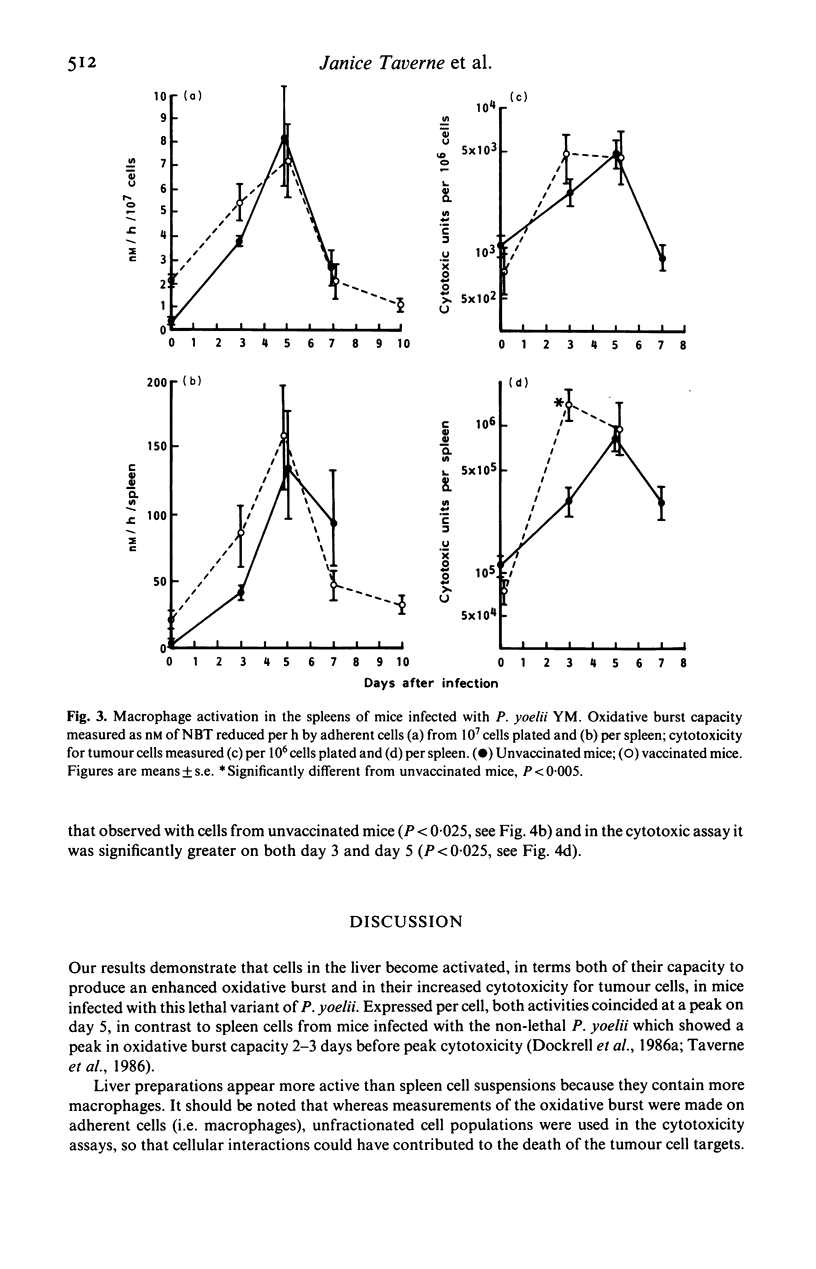
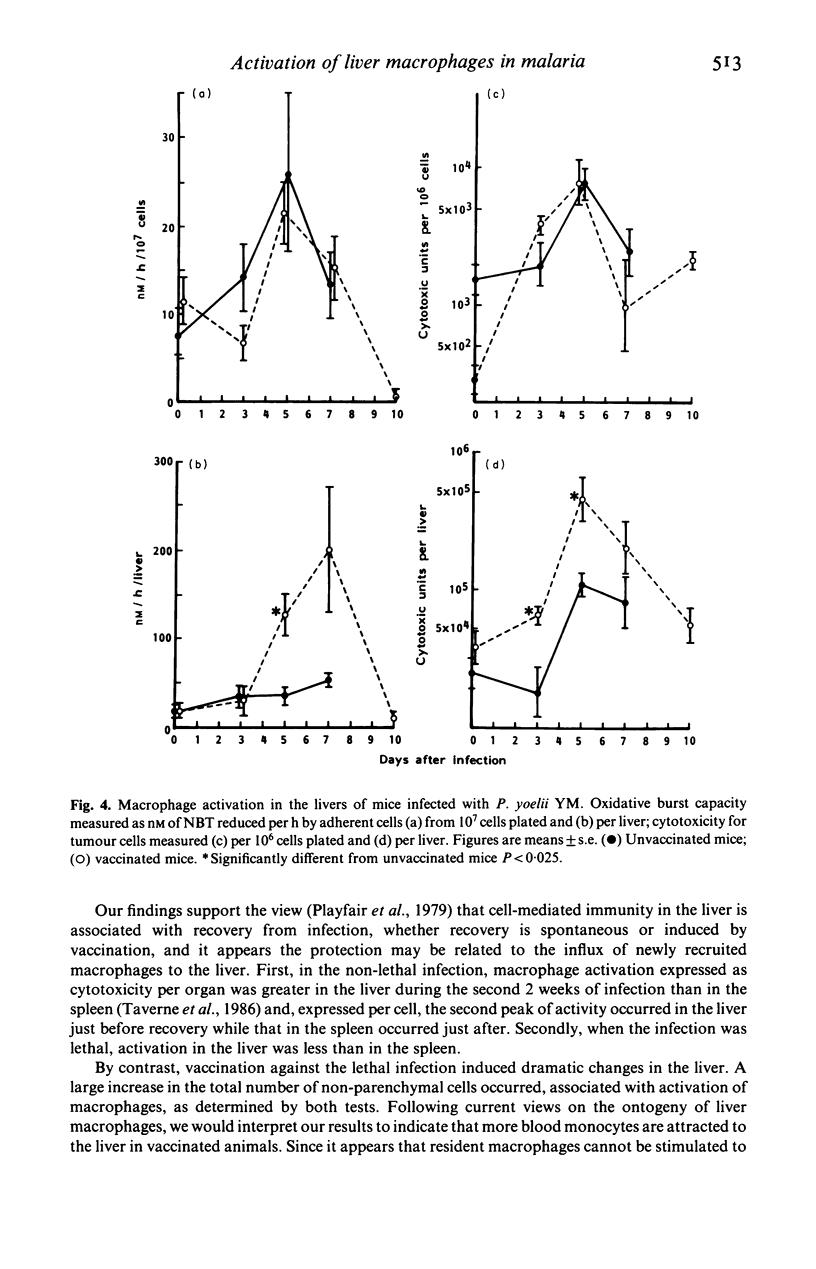

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M. J., Kowalski-Saunders P., Wright R. Corynebacterium parvum-elicited hepatic macrophages demonstrate enhanced respiratory burst activity compared with resident Kupffer cells in the rat. Gastroenterology. 1986 Jul;91(1):174–181. doi: 10.1016/0016-5085(86)90455-5. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Kaufmann S. H., Simon M. M., Fischer H. Role of macrophages in malaria: O2 metabolite production and phagocytosis by splenic macrophages during lethal Plasmodium berghei and self-limiting Plasmodium yoelii infection in mice. Infect Immun. 1984 Jun;44(3):743–746. doi: 10.1128/iai.44.3.743-746.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Blackwell J. M., Bradley D. J. Expression of the natural resistance gene Lsh in resident liver macrophages. Infect Immun. 1984 Mar;43(3):1033–1040. doi: 10.1128/iai.43.3.1033-1040.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Alavi A., Playfair J. H. Changes in oxidative burst capacity during murine malaria and the effect of vaccination. Clin Exp Immunol. 1986 Oct;66(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of Plasmodium yoelii by enzyme-induced products of the oxidative burst. Infect Immun. 1984 Feb;43(2):451–456. doi: 10.1128/iai.43.2.451-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., de Souza J. B., Playfair J. H. The role of the liver in immunity to blood-stage murine malaria. Immunology. 1980 Oct;41(2):421–430. [PMC free article] [PubMed] [Google Scholar]
- Freeman R. R., Holder A. A. Characteristics of the protective response of BALB/c mice immunized with a purified Plasmodium yoelii schizont antigen. Clin Exp Immunol. 1983 Dec;54(3):609–616. [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Crocker P., Gordon S. Macrophage plasma membrane and secretory properties in murine malaria. Effects of Plasmodium yoelii blood-stage infection on macrophages in liver, spleen, and blood. J Exp Med. 1986 Jan 1;163(1):54–74. doi: 10.1084/jem.163.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepay D. A., Nathan C. F., Steinman R. M., Murray H. W., Cohn Z. A. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J Exp Med. 1985 May 1;161(5):1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepay D. A., Steinman R. M., Nathan C. F., Murray H. W., Cohn Z. A. Liver macrophages in murine listeriosis. Cell-mediated immunity is correlated with an influx of macrophages capable of generating reactive oxygen intermediates. J Exp Med. 1985 Jun 1;161(6):1503–1512. doi: 10.1084/jem.161.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Shear H. L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J Immunol. 1984 Jan;132(1):424–431. [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Cottrell B. J. Protection of mice against malaria by a killed vaccine: differences in effectiveness against P. yoelii and P. berghei. Immunology. 1977 Oct;33(4):507–515. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Dockrell H. M., Agomo P. U., Taverne J. Cell-mediated immunity in the liver of mice vaccinated against malaria. Nature. 1979 Dec 13;282(5740):731–734. doi: 10.1038/282731a0. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., de Souza J. B. Lymphocyte traffic and lymphocyte destruction in murine malaria. Immunology. 1982 May;46(1):125–133. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Umar S., Dockrell H. M. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by gamma-interferon. J Immunol Methods. 1985 Sep 3;82(1):161–167. doi: 10.1016/0022-1759(85)90235-2. [DOI] [PubMed] [Google Scholar]


