Abstract
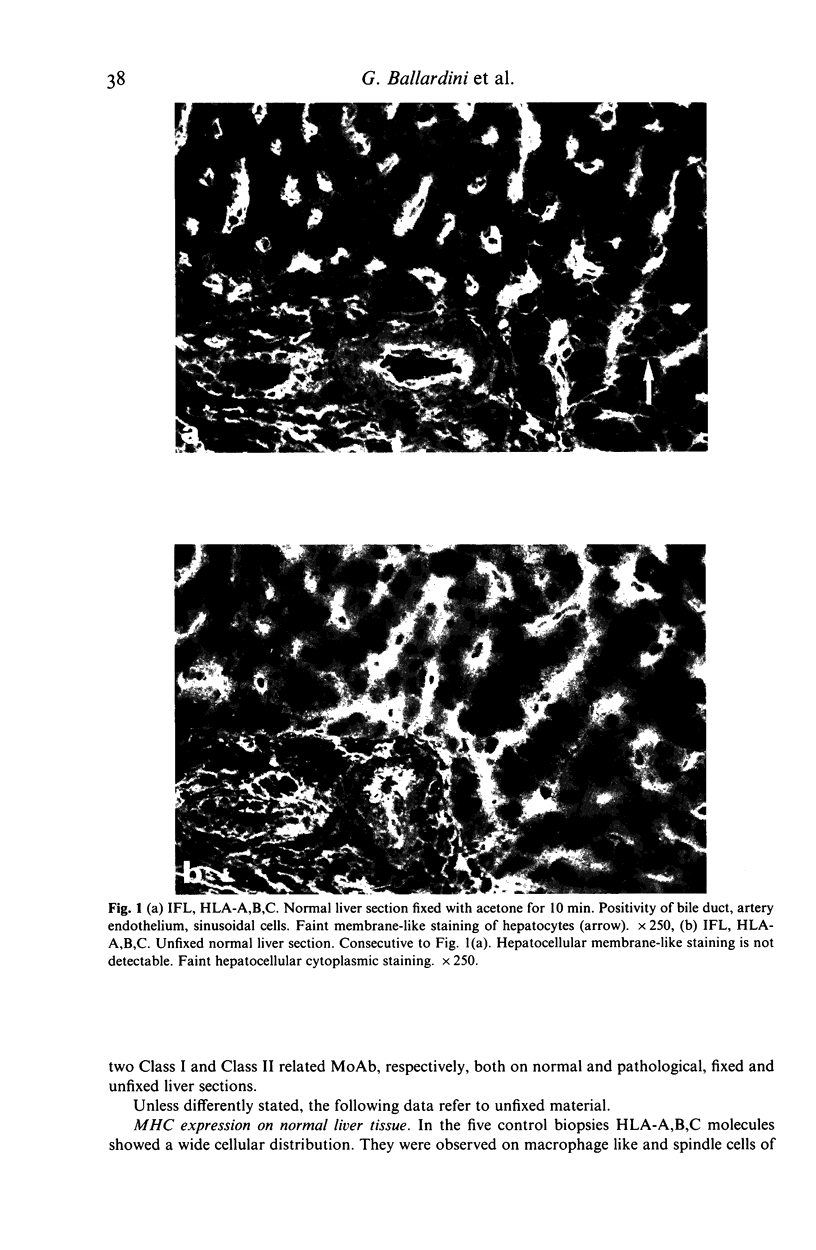
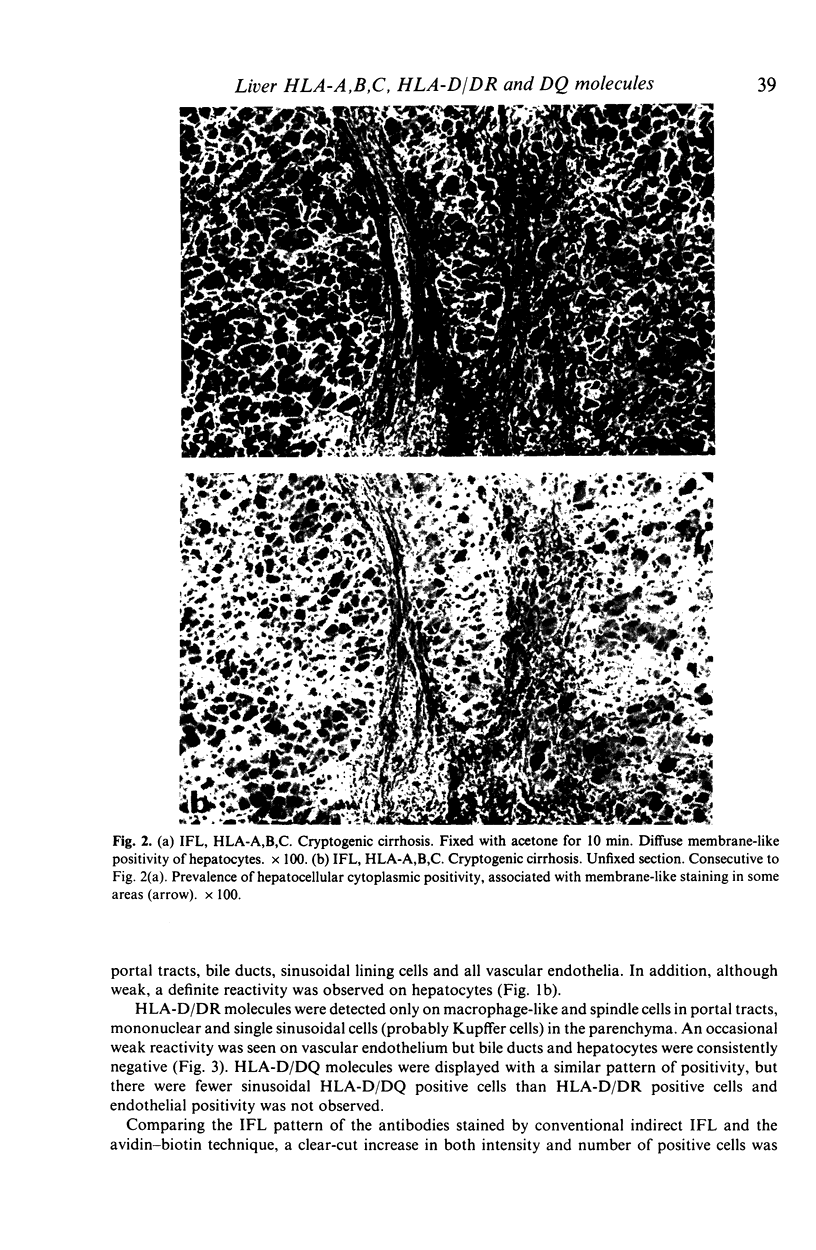
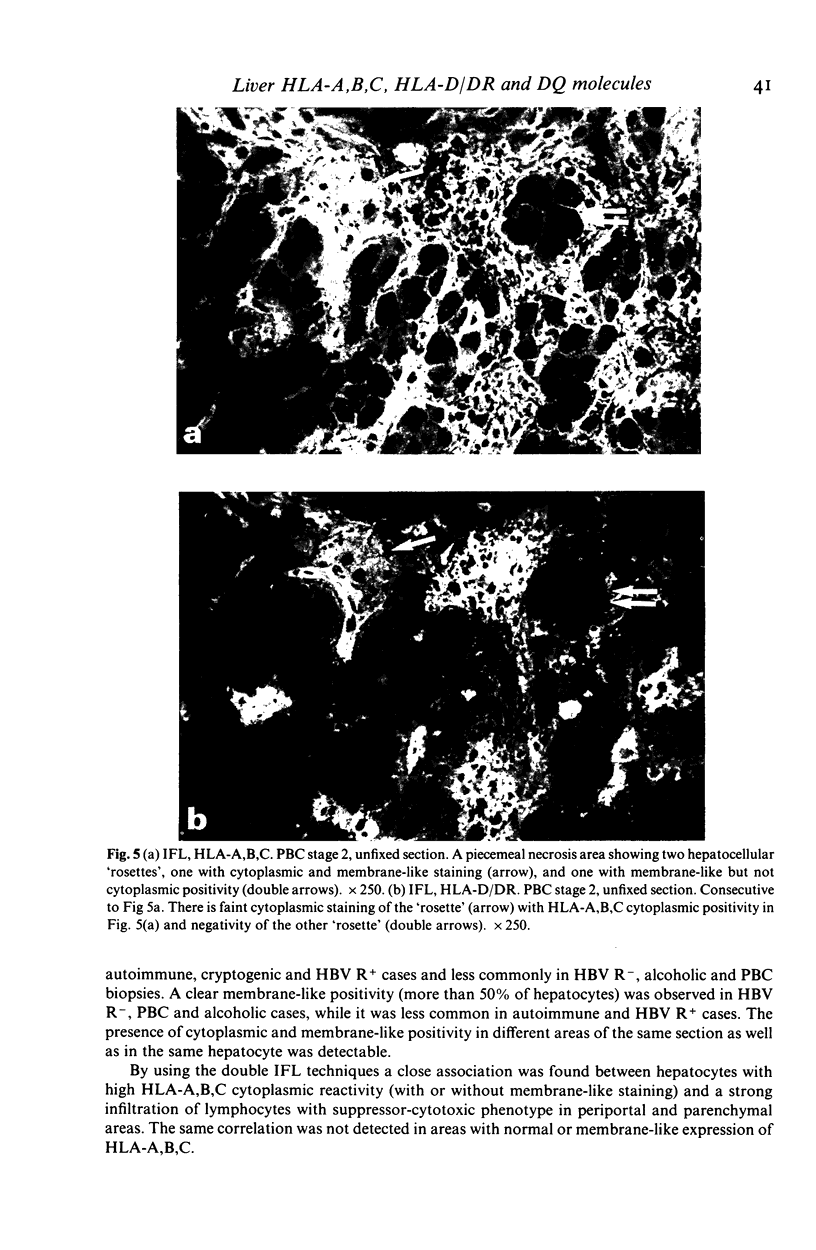
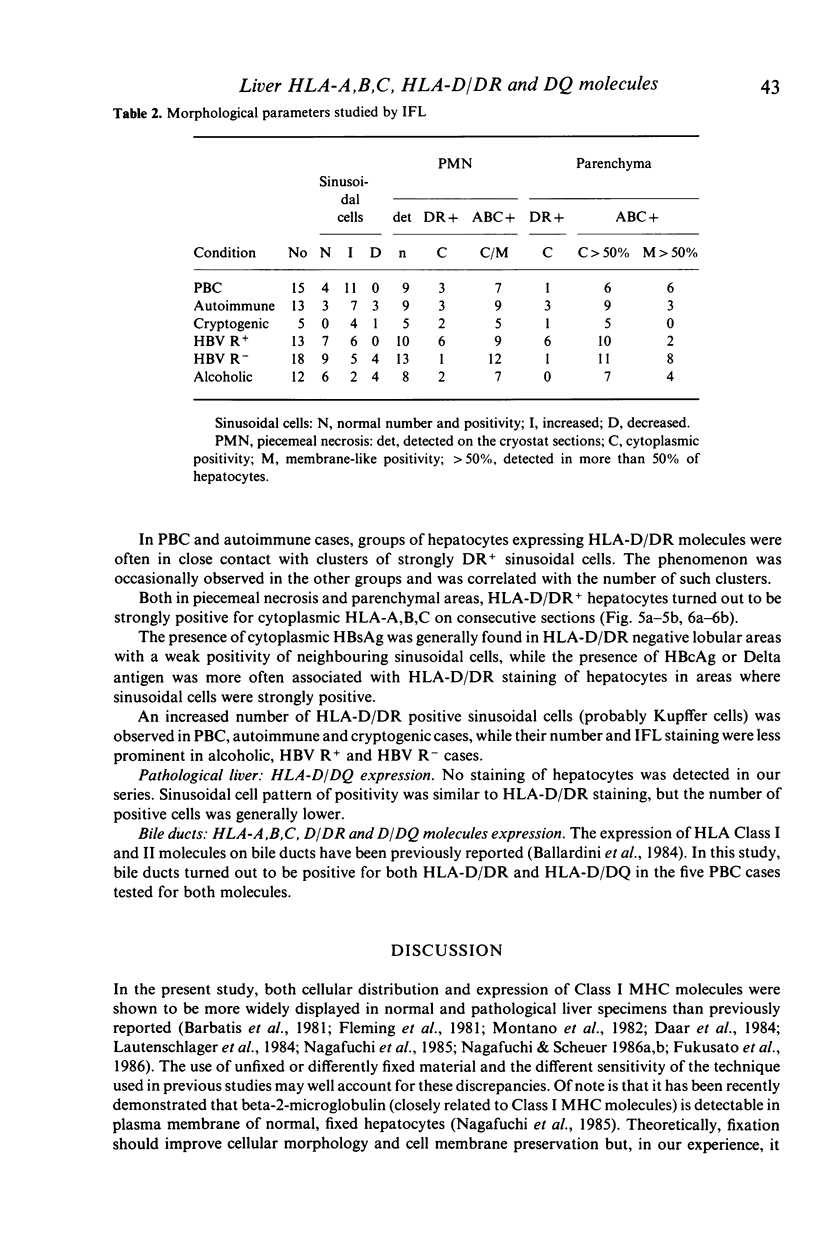
The distribution of HLA-A,B,C, HLA-D/DR and HLA-D/DQ molecules was studied by indirect immunofluorescence with an avidin-biotin technique and monoclonal antibodies, in unfixed cryostat sections of liver biopsies from 76 patients with chronic liver diseases of various aetiologies and five normal liver biopsy specimens. In pathological liver, strong cytoplasmic or membrane-like positivity for HLA-A,B,C of hepatocytes was observed in piecemeal necrosis areas in all groups. Cytoplasmic staining was mainly seen in lobular areas in autoimmune, cryptogenic and HBV-related cases with viral replication, while membrane-like positivity was more frequently observed in primary biliary cirrhosis, alcoholic and HBV-related cases without viral replication. A weak cytoplasmic staining for HLA-D/DR was observed in piecemeal necrosis and lobular areas mainly in HBV-related cases with viral replication. While bile duct cells were positive for both HLA-D/DR and HLA-D/DQ, hepatocytes were consistently HLA-D/DQ negative. The increased HLA-A,B,C expression on hepatocytes should allow T cytotoxic cell aggression. Hepatocellular HLA-D/DR expression is definite but weak and probably does not allow direct autoantigen presentation and induction of autoimmunity. Negativity for HLA-D/DQ further supports this hypothesis. Since cytoplasmic staining for Class I and II molecules is greatly lowered by fixing cryostat liver sections, prestaining conditions should be taken into account when comparing different studies.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander G., Williams R. Characterization of the mononuclear cell infiltrate in piecemeal necrosis. Lab Invest. 1984 Mar;50(3):247–249. [PubMed] [Google Scholar]
- Ballardini G., Mirakian R., Bianchi F. B., Pisi E., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigens on bileduct epithelium in primary biliary cirrhosis: relevance to pathogenesis. Lancet. 1984 Nov 3;2(8410):1009–1013. doi: 10.1016/s0140-6736(84)91108-5. [DOI] [PubMed] [Google Scholar]
- Barbatis C., Woods J., Morton J. A., Fleming K. A., McMichael A., McGee J. O. Immunohistochemical analysis of HLA (A, B, C) antigens in liver disease using a monoclonal antibody. Gut. 1981 Dec;22(12):985–991. doi: 10.1136/gut.22.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Daar A. S., Fuggle S. V., Fabre J. W., Ting A., Morris P. J. The detailed distribution of HLA-A, B, C antigens in normal human organs. Transplantation. 1984 Sep;38(3):287–292. doi: 10.1097/00007890-198409000-00018. [DOI] [PubMed] [Google Scholar]
- Fleming K. A., McMichael A., Morton J. A., Woods J., McGee J. O. Distribution of HLA class 1 antigens in normal human tissue and in mammary cancer. J Clin Pathol. 1981 Jul;34(7):779–784. doi: 10.1136/jcp.34.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukusato T., Gerber M. A., Thung S. N., Ferrone S., Schaffner F. Expression of HLA class I antigens on hepatocytes in liver disease. Am J Pathol. 1986 May;123(2):264–270. [PMC free article] [PubMed] [Google Scholar]
- Gonwa T. A., Picker L. J., Raff H. V., Goyert S. M., Silver J., Stobo J. D. Antigen-presenting capabilities of human monocytes correlates with their expression of HLA-DS, an Ia determinant distinct from HLA-DR. J Immunol. 1983 Feb;130(2):706–711. [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager I., Taskinen E., Inkinen K., Lehto V. P., Virtanen I., Häyry P. Distribution of the major histocompatibility complex antigens on different cellular components of human liver. Cell Immunol. 1984 Apr 15;85(1):191–200. doi: 10.1016/0008-8749(84)90289-2. [DOI] [PubMed] [Google Scholar]
- Montano L., Miescher G. C., Goodall A. H., Wiedmann K. H., Janossy G., Thomas H. C. Hepatitis B virus and HLA antigen display in the liver during chronic hepatitis B virus infection. Hepatology. 1982 Sep-Oct;2(5):557–561. doi: 10.1002/hep.1840020508. [DOI] [PubMed] [Google Scholar]
- Möst J., Knapp W., Wick G. Class II antigens in Hashimoto thyroiditis. I. Synthesis and expression of HLA-DR and HLA-DQ by thyroid epithelial cells. Clin Immunol Immunopathol. 1986 Nov;41(2):165–174. doi: 10.1016/0090-1229(86)90100-5. [DOI] [PubMed] [Google Scholar]
- Nagafuchi Y., Hobbs K. E., Thomas H. C., Scheuer P. J. Expression of beta-2-microglobulin on hepatocytes after liver transplantation. Lancet. 1985 Mar 9;1(8428):551–554. doi: 10.1016/s0140-6736(85)91209-7. [DOI] [PubMed] [Google Scholar]
- Nagafuchi Y., Scheuer P. J. Expression of beta 2-microglobulin on hepatocytes in acute and chronic type B hepatitis. Hepatology. 1986 Jan-Feb;6(1):20–23. doi: 10.1002/hep.1840060105. [DOI] [PubMed] [Google Scholar]
- Nagafuchi Y., Scheuer P. J. Hepatic beta 2-microglobulin distribution in primary biliary cirrhosis. J Hepatol. 1986;2(1):73–80. doi: 10.1016/s0168-8278(86)80010-1. [DOI] [PubMed] [Google Scholar]
- Natali P. G., De Martino C., Quaranta V., Nicotra M. R., Frezza F., Pellegrino M. A., Ferrone S. Expression of Ia-like antigens in normal human nonlymphoid tissues. Transplantation. 1981 Jan;31(1):75–78. doi: 10.1097/00007890-198101000-00017. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Segatto O., Ferrone S., Tosi R., Corte G. Differential tissue distribution and ontogeny of DC-1 and HLA-DR antigens. Immunogenetics. 1984;19(2):109–116. doi: 10.1007/BF00387853. [DOI] [PubMed] [Google Scholar]
- Pignatelli M., Waters J., Brown D., Lever A., Iwarson S., Schaff Z., Gerety R., Thomas H. C. HLA class I antigens on the hepatocyte membrane during recovery from acute hepatitis B virus infection and during interferon therapy in chronic hepatitis B virus infection. Hepatology. 1986 May-Jun;6(3):349–353. doi: 10.1002/hep.1840060303. [DOI] [PubMed] [Google Scholar]
- Thomas H. C., Pignatelli M. Is modulation of HLA display by interferon important in preventing the development of the chronic carrier state of hepatitis B virus in adults? Gastroenterol Clin Biol. 1985 Apr;9(4):287–289. [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981 Oct;29(10):1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zoppi G., Gasparini R., Mantovanelli F., Gobio-Casali L., Astolfi R., Crovari P. Diet and antibody response to vaccinations in healthy infants. Lancet. 1983 Jul 2;2(8340):11–14. doi: 10.1016/s0140-6736(83)90004-1. [DOI] [PubMed] [Google Scholar]



