Abstract
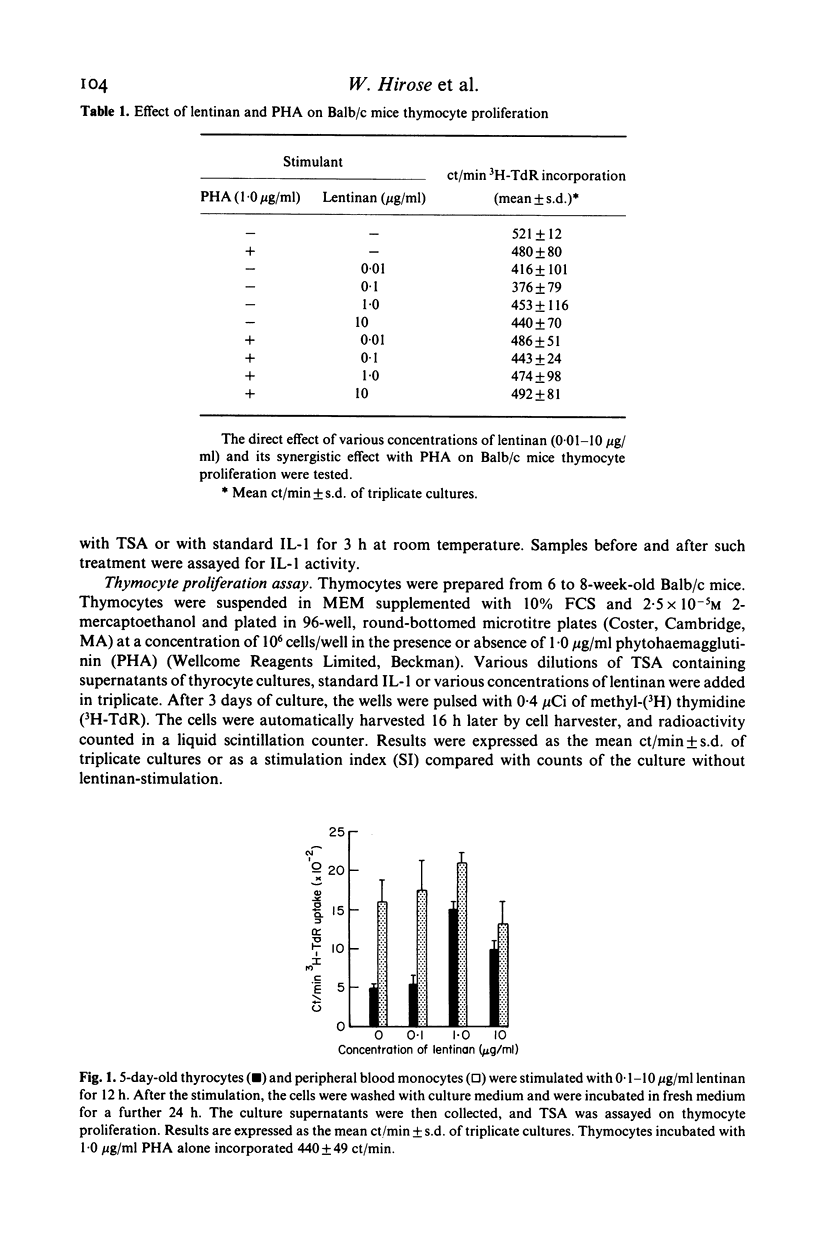
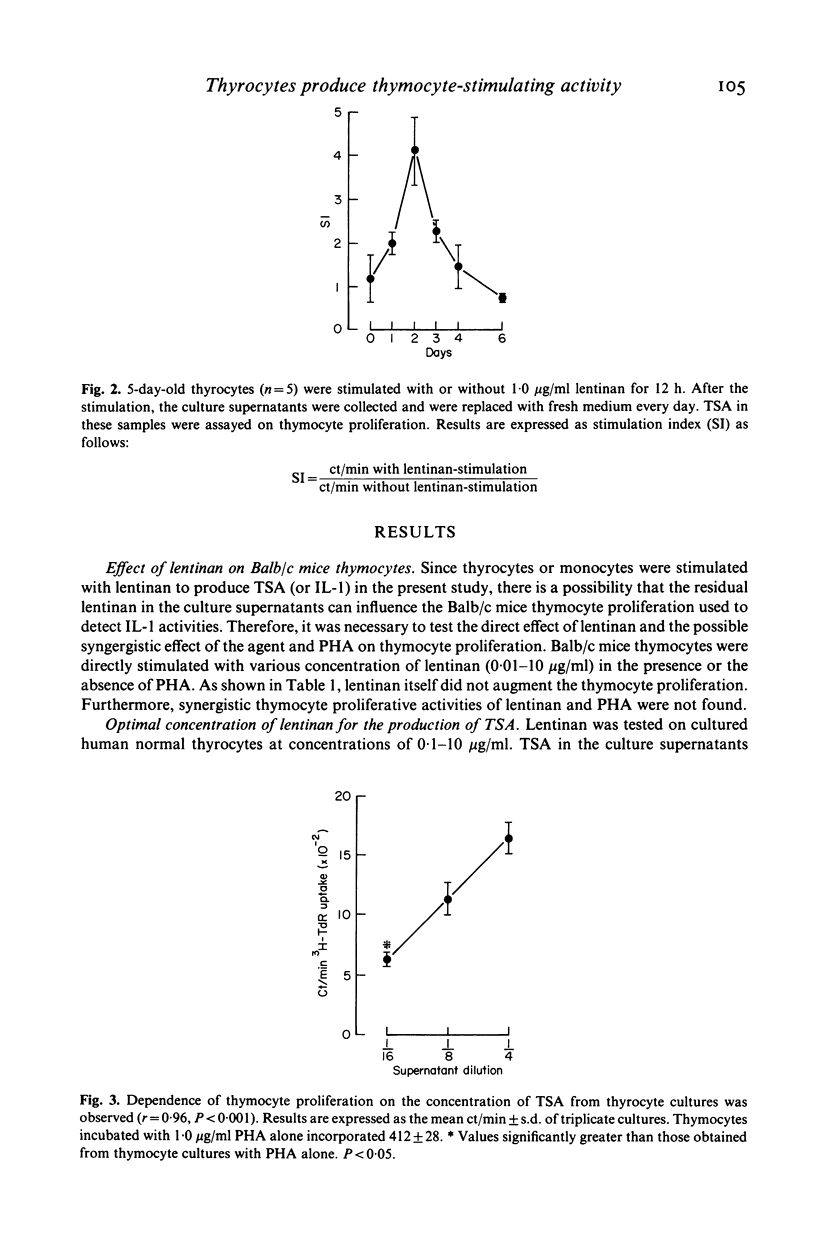
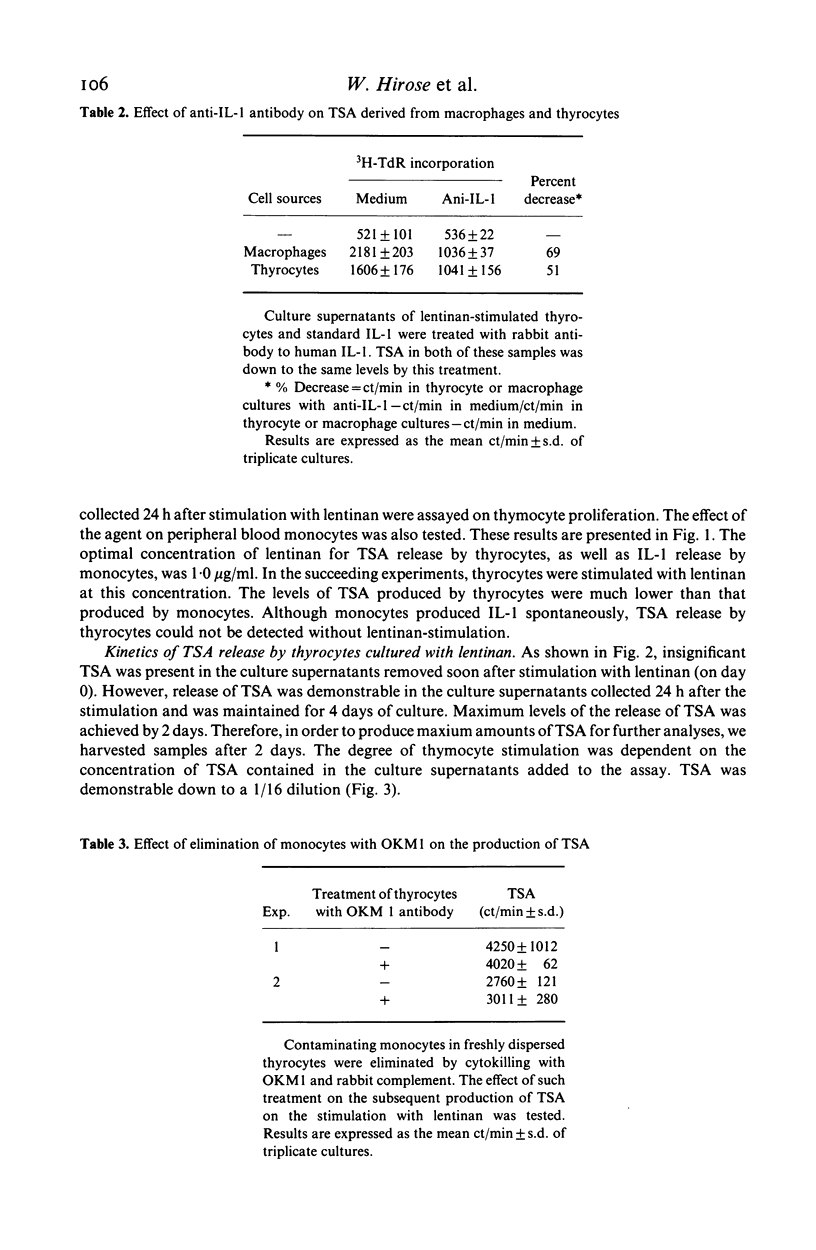
Since thyroid follicular epithelial cells (thyrocytes) have been shown to express a number of functions similar to monocytes, they were further examined for their potency in secreting thymocyte-stimulating activity (TSA). Although spontaneous production of TSA could not be detected when thyrocytes were cultured in the culture medium, TSA was demonstrated in the culture supernatants after stimulation with the immune adjuvant lentinan. The release of TSA was found in the culture supernatants collected 24 h after stimulation and was maintained for the 4 days of culture. Maximum levels of TSA release were achieved by 2 days. In addition, when culture supernatants of thyrocytes stimulated with lentinan or monocyte-derived interleukin 1 (IL-1) were incubated with a rabbit antibody to human IL-1, a parallel reduction in TSA was observed, suggesting that the active product in the thyrocyte culture supernatant shared a common antigenic site with IL-1. The demonstration of the production of IL-1 like activity by thyrocytes provides additional evidence that these cells, in addition to their functions as endocrine cells, may also participate in the local immune responses under appropriate conditions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berle E. J., Jr, Thorsby E. The proliferative T cell response to herpes simplex virus (HSV) antigen is restricted by self HLA-D. Clin Exp Immunol. 1980 Mar;39(3):668–675. [PMC free article] [PubMed] [Google Scholar]
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Chihara G., Hamuro J., Maeda Y., Arai Y., Fukuoka F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk.) Sing. (an edible mushroom). Cancer Res. 1970 Nov;30(11):2776–2781. [PubMed] [Google Scholar]
- Chu E. T., Lareau M., Rosenwasser L. J., Dinarello C. A., Geha R. S. Antigen presentation by EBV-B cells to resting and activated T cells: role of interleukin 1. J Immunol. 1985 Mar;134(3):1676–1681. [PubMed] [Google Scholar]
- Davies T. F. Cocultures of human thyroid monolayer cells and autologous T cells: impact of HLA class II antigen expression. J Clin Endocrinol Metab. 1985 Sep;61(3):418–422. doi: 10.1210/jcem-61-3-418. [DOI] [PubMed] [Google Scholar]
- Di Luzio N. R., Williams D. L., McNamee R. B., Edwards B. F., Kitahama A. Comparative tumor-inhibitory and anti-bacterial activity of soluble and particulate glucan. Int J Cancer. 1979 Dec 15;24(6):773–779. doi: 10.1002/ijc.2910240613. [DOI] [PubMed] [Google Scholar]
- Fruehauf J. P., Bonnard G. D., Herberman R. B. The effect of lentinan on production of interleukin-1 by human monocytes. Immunopharmacology. 1982 Oct;5(1):65–74. doi: 10.1016/0162-3109(82)90037-6. [DOI] [PubMed] [Google Scholar]
- Hamuro J., Röllinghoff M., Wagner H. Induction of cytotoxic peritoneal exudate cells by T-cell immune adjuvants of the beta(1 leads to 3) glucan-type lentinan and its analogues. Immunology. 1980 Apr;39(4):551–559. [PMC free article] [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Jacquemont C., Pruniéras M. Culture de longue durée de cellules issues de l'épiderme de cobaye adulte. II. Essai de caractérisation cytoenzymologique. Pathol Biol (Paris) 1969 Mar;17(5):243–249. [PubMed] [Google Scholar]
- Jansson R., Karlsson A., Forsum U. Intrathyroidal HLA-DR expression and T lymphocyte phenotypes in Graves' thyrotoxicosis, Hashimoto's thyroiditis and nodular colloid goitre. Clin Exp Immunol. 1984 Nov;58(2):264–272. [PMC free article] [PubMed] [Google Scholar]
- Khoury E. L., Hammond L., Bottazzo G. F., Doniach D. Presence of the organ-specific 'microsomal' autoantigen on the surface of human thyroid cells in culture: its involvement in complement-mediated cytotoxicity. Clin Exp Immunol. 1981 Aug;45(2):316–328. [PMC free article] [PubMed] [Google Scholar]
- Kurnick J. T., Grönvik K. O., Kimura A. K., Lindblom J. B., Skoog V. T., Sjöberg O., Wigzell H. Long term growth in vitro of human T cell blasts with maintenance of specificity and function. J Immunol. 1979 Apr;122(4):1255–1260. [PubMed] [Google Scholar]
- Lepe-Zuniga J. L., Gery I. Production of intra- and extracellular interleukin-1 (IL-1) by human monocytes. Clin Immunol Immunopathol. 1984 May;31(2):222–230. doi: 10.1016/0090-1229(84)90242-3. [DOI] [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Londei M., Lamb J. R., Bottazzo G. F., Feldmann M. Epithelial cells expressing aberrant MHC class II determinants can present antigen to cloned human T cells. Nature. 1984 Dec 13;312(5995):639–641. doi: 10.1038/312639a0. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Ryan J. L., Sterzel R. B. A thymocyte-activating factor derived from glomerular mesangial cells. J Immunol. 1983 Apr;130(4):1796–1801. [PubMed] [Google Scholar]
- Luger T. A., Stadler B. M., Katz S. I., Oppenheim J. J. Epidermal cell (keratinocyte)-derived thymocyte-activating factor (ETAF). J Immunol. 1981 Oct;127(4):1493–1498. [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Production of interleukin 1 by human endothelial cells. J Immunol. 1986 Apr 1;136(7):2486–2491. [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Piccinini L. A., Mackenzie W. A., Platzer M., Davies T. F. Lymphokine regulation of HLA-DR gene expression in human thyroid cell monolayers. J Clin Endocrinol Metab. 1987 Mar;64(3):543–548. doi: 10.1210/jcem-64-3-543. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]


