Abstract
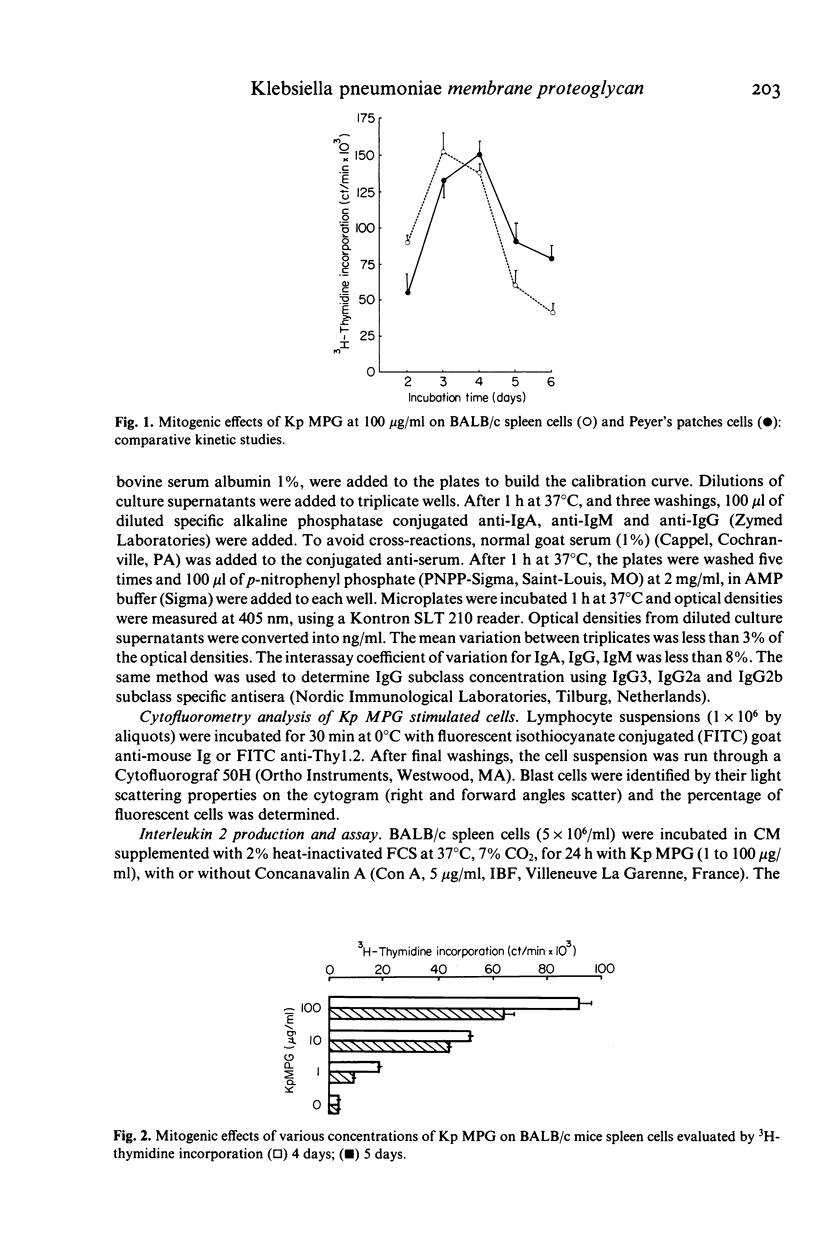
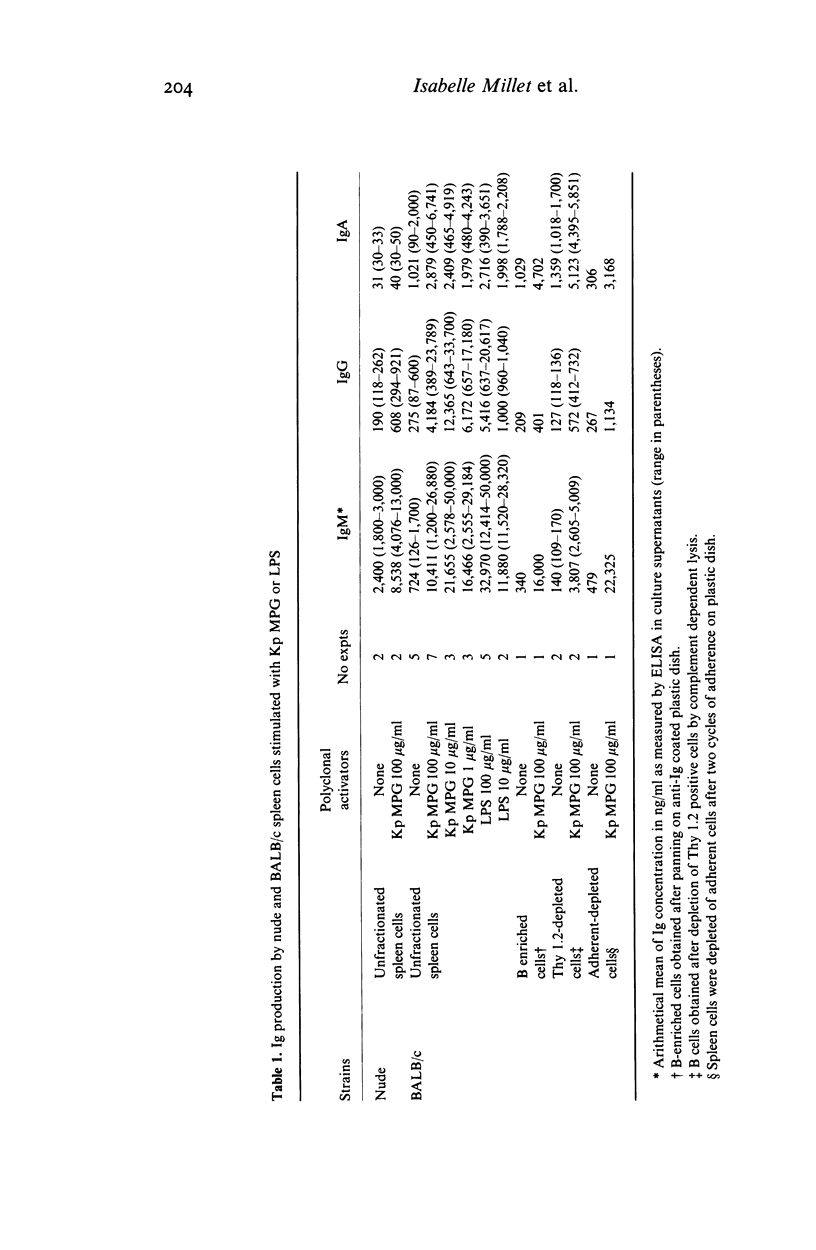
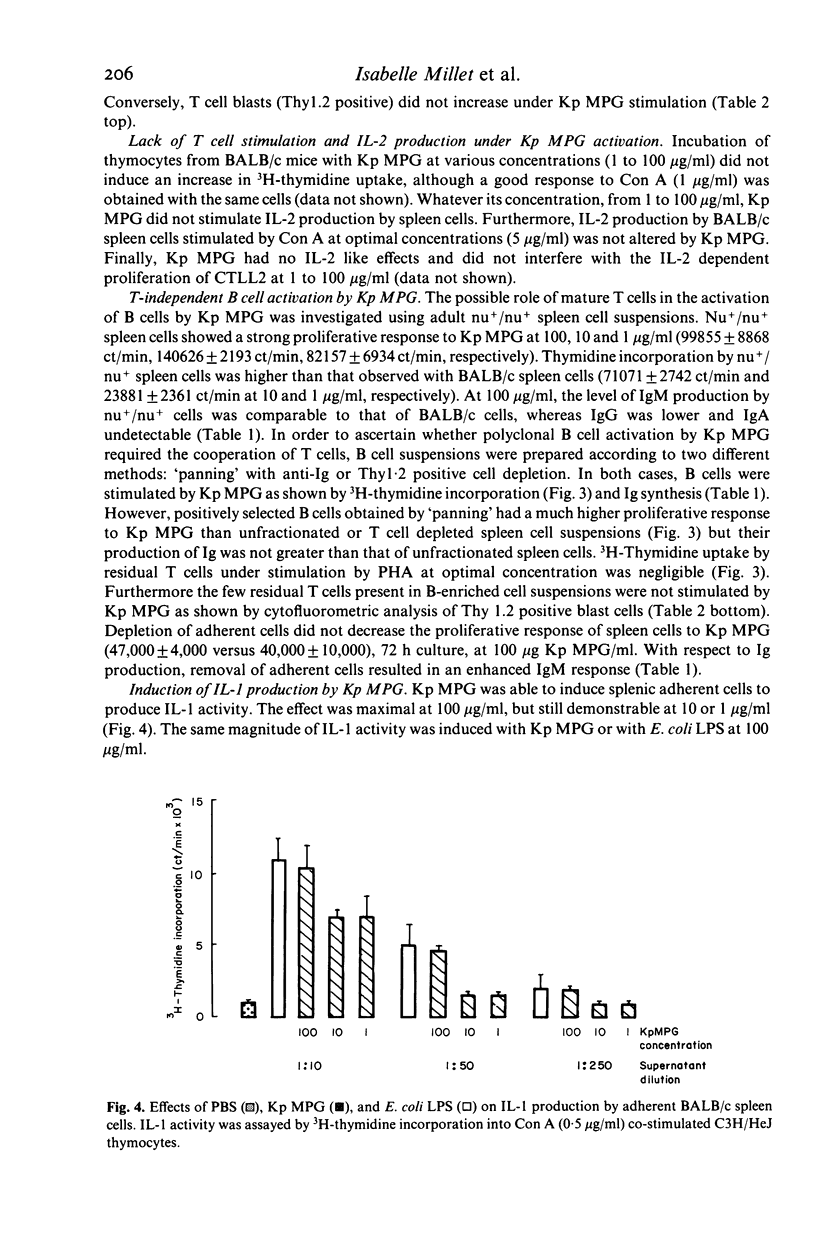
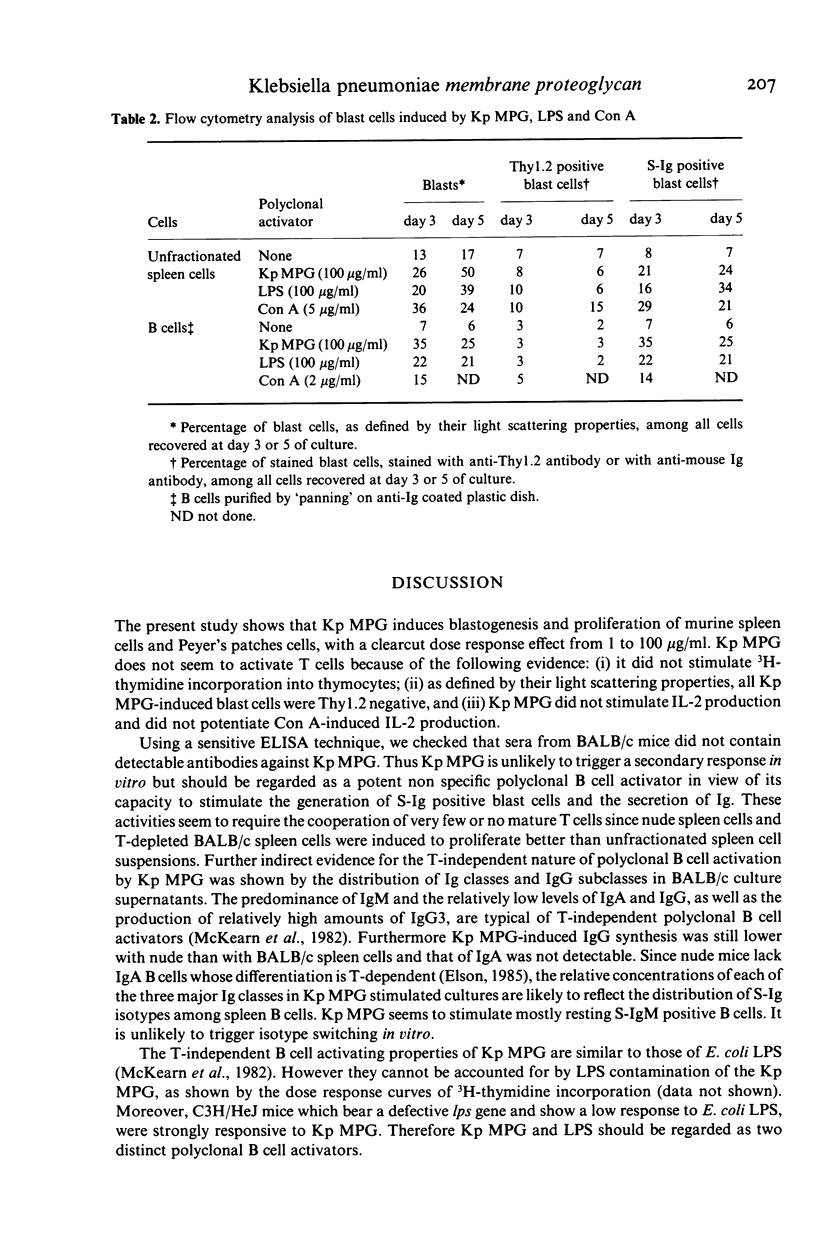
The lymphocyte activating properties of a membrane proteoglycan (MPG) extracted from a mutant non-encapsulated strain of Klebsiella pneumoniae (Kp) (biotype a I-145) were investigated. Kp MPG induced a strong proliferative response of BALB/c spleen cells and Peyer's patches cells. Thymidine incorporation was dose-related (from 1 to 100 micrograms Kp MPG/ml) and reached a maximum at day 3. It was not reduced by removal of most adherent cells, nor by depletion of Thy1-2 positive cells, but it was abrogated by removal of surface immunoglobulin bearing cells. Spleen cells from nude mice and those from C3H/Hej mice were strongly stimulated by Kp MPG. Conversely Kp MPG did not induce interleukin 2 production and did not trigger the proliferation of thymocytes but stimulated interleukin 1 production by adherent spleen cells. Finally, unfractionated or B-enriched spleen cells cultured with Kp MPG synthesized IgM and, to a lesser extent, IgG and IgA. It is concluded that Kp MPG is a T-independent polyclonal B cell activator and an inducer of interleukin 1 production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barot-Ciorbaru R., Brochier J., Miyawaki T., Preud'Homme J. L., Petit J. F., Bona C., Taniguchi N., Revillard J. P. Stimulation of human B lymphocytes by Nocardia-delipidated cell mitogen and derived fractions from N. opaca. Structure-activity relationship. J Immunol. 1985 Nov;135(5):3277–3283. [PubMed] [Google Scholar]
- Damais C., Bona C., Chedid L., Fleck J., Nauciel C., Martin J. P. Mitogenic effect of bacterial peptidoglycans possessing adjuvant activity. J Immunol. 1975 Jul;115(1):268–271. [PubMed] [Google Scholar]
- Defranco A. L., Raveche E. S., Asofsky R., Paul W. E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982 May 1;155(5):1523–1536. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussourd d'Hinterland L., Normier G., Durand J. Ribosomal vaccines: preparation of subcellular fractions. Arzneimittelforschung. 1980;30(1A):126–132. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Howard M., Paul W. E. Regulation of B-cell growth and differentiation by soluble factors. Annu Rev Immunol. 1983;1:307–333. doi: 10.1146/annurev.iy.01.040183.001515. [DOI] [PubMed] [Google Scholar]
- McKearn J. P., Paslay J. W., Slack J., Baum C., Davie J. M. B cell subsets and differential responses to mitogens. Immunol Rev. 1982;64:5–23. doi: 10.1111/j.1600-065x.1982.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Michel F. B., Dussourd D'Hinterland L., Bousquet J., Pinel A. M., Normier G. Immuno-stimulation by a ribosomal vaccine associated with a bacterial cell wall adjuvant in humans. Infect Immun. 1978 Jun;20(3):760–769. doi: 10.1128/iai.20.3.760-769.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B., Mizel D. Purification to apparent homogeneity of murine interleukin 1. J Immunol. 1981 Mar;126(3):834–837. [PubMed] [Google Scholar]
- Nakashima I., Kato N. Non-specific stimulation of immunoglobulin synthesis in mice by capsular polysaccharide of Klebsiella pneumoniae. Immunology. 1974 Aug;27(2):179–193. [PMC free article] [PubMed] [Google Scholar]
- Robert D., Bienvenu P., Lafont S., Jouanneteau B., Normier G., Dussourd D'Hinterland L., Fontanges R. An attempt to localize the vaccinating power of Klebsiella pneumoniae ribosomal preparations using saccharose-gradient ultracentrifugation. Microbiol Immunol. 1982;26(10):941–950. doi: 10.1111/j.1348-0421.1982.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Räsänen L., Arvilommi H. Cell walls, peptidoglycans, and teichoic acids of gram-positive bacteria as polyclonal inducers and immunomodulators of proliferative and lymphokine responses of human B and T lymphocytes. Infect Immun. 1981 Dec;34(3):712–717. doi: 10.1128/iai.34.3.712-717.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kotani S., Kusumoto S., Inage M., Shiba T., Nagao S., Yano I., Kawata S., Yokogawa K. Mitogenic effects of bacterial cell walls, their fragments, and related synthetic compounds on thymocytes and splenocytes of guinea pigs. Infect Immun. 1979 Aug;25(2):645–652. doi: 10.1128/iai.25.2.645-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S. I., Wannemuehler M. J., Jirillo E., Pritchard D. G., Michalek S. M., McGhee J. R. LPS regulation of the immune response: separate mechanisms for murine B cell activation by lipid A (direct) and polysaccharide (macrophage-dependent) derived from Bacteroides LPS. J Immunol. 1984 Nov;133(5):2294–2300. [PubMed] [Google Scholar]
- Wood C. D., Möller G. Influence of RU 41.740, a glycoprotein extract from Klebsiella pneumoniae, on the murine immune system. I. T-independent polyclonal B cell activation. J Immunol. 1984 Feb;132(2):616–621. [PubMed] [Google Scholar]


