Abstract
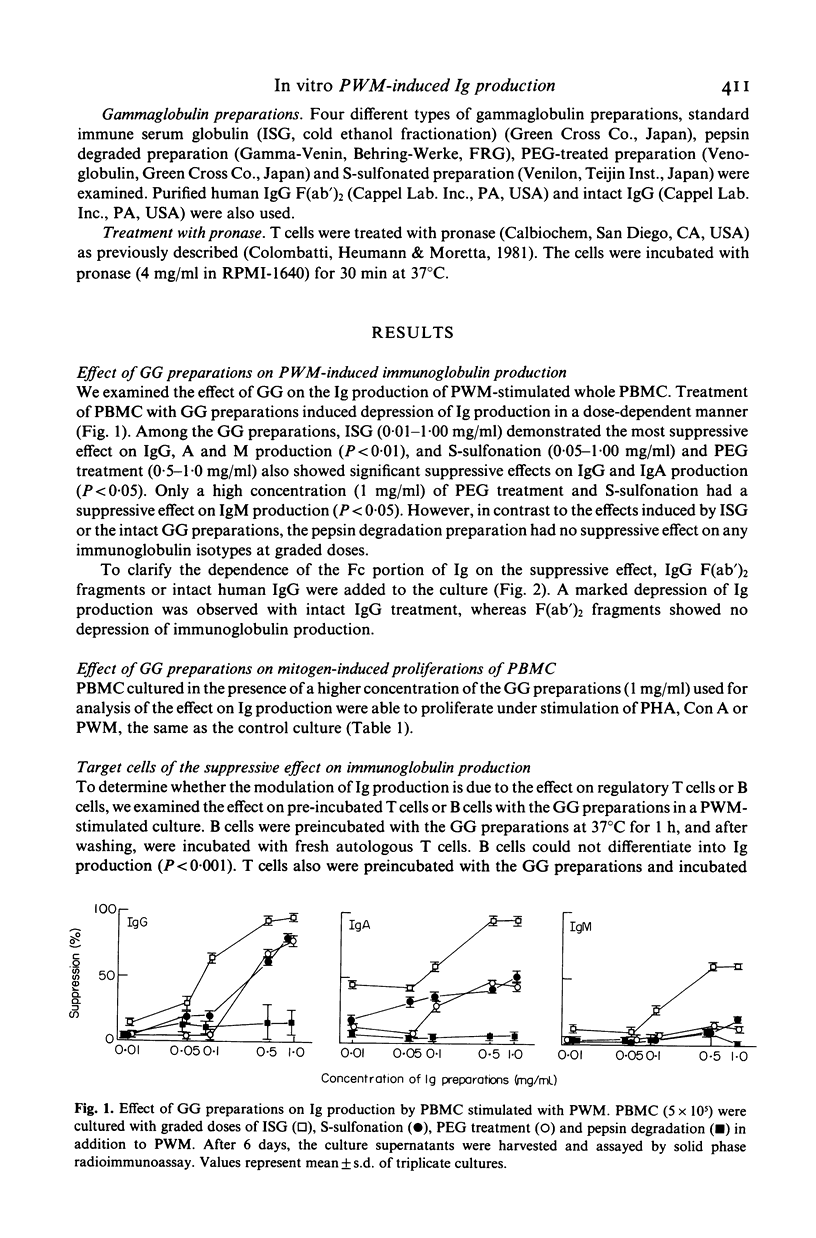
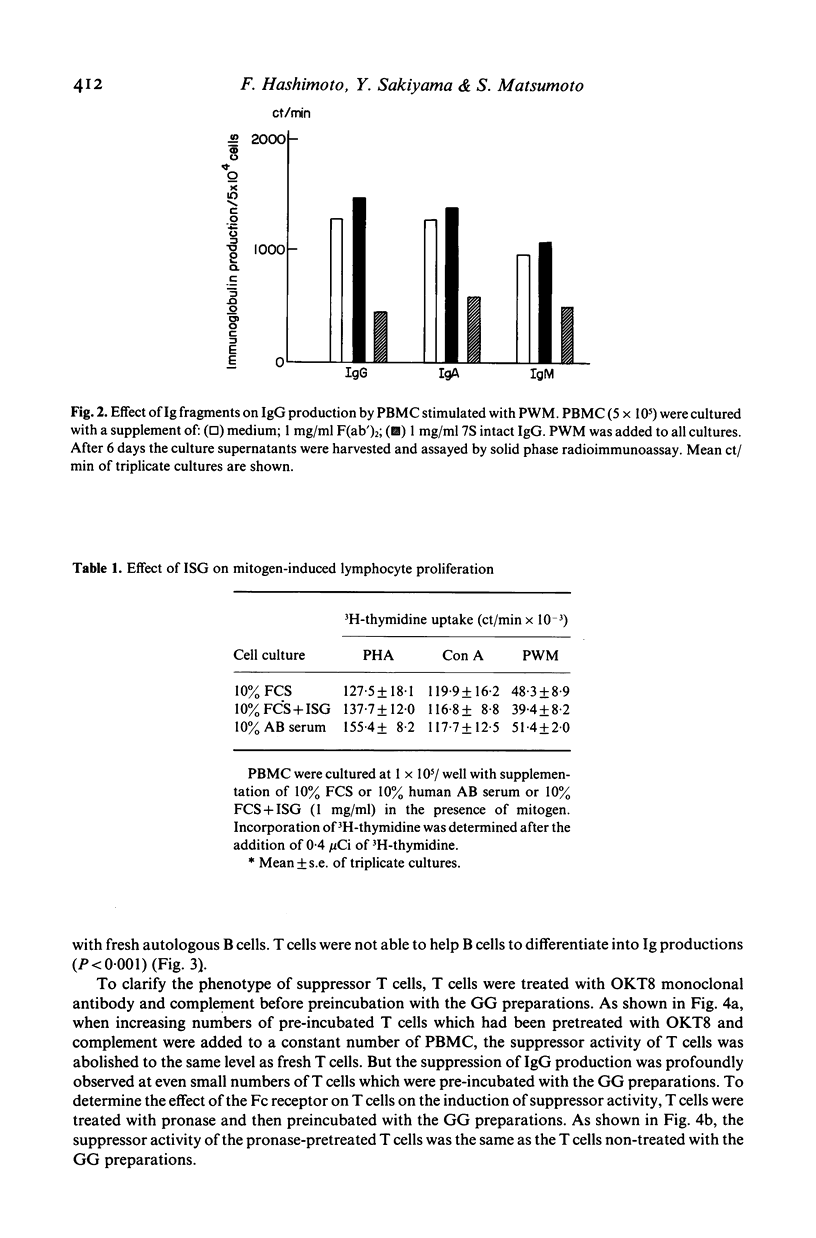
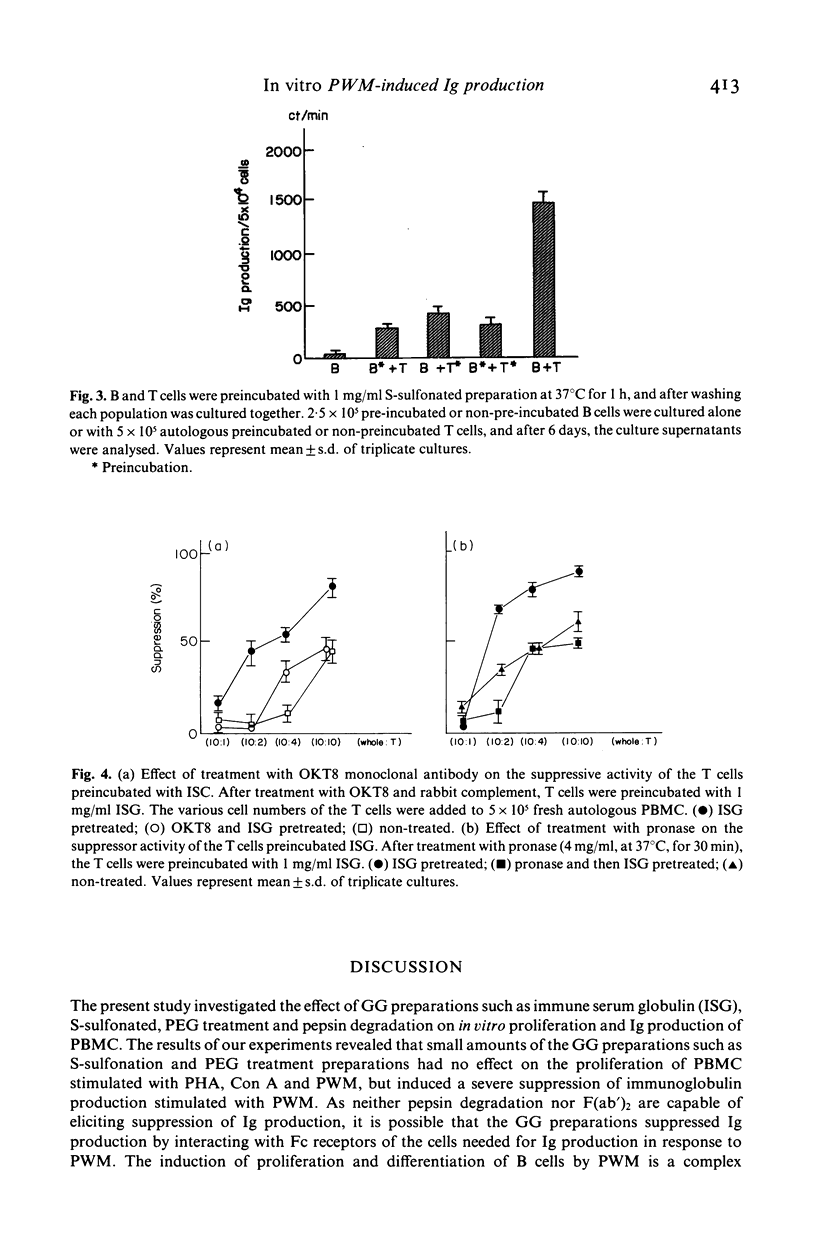
The effect of the supplementation with several gammaglobulin (GG) preparations on the in vitro immunoglobulin synthesis of peripheral blood mononuclear cells (PBMC) from normal subjects stimulated with pokeweed mitogen (PWM) was studied. Among the GG preparations used in this study, immune serum globulin (ISG) demonstrated the most suppressive effect, and S-sulfonation and polyethylene glycol (PEG)-treated preparations also had a suppressive effect. However, the preparation of pepsin degradation had no suppressive effect. And because IgG F(ab')2 fragments also failed to induce the suppressive effect, it was considered to be triggered by the attachment of the Fc portion of GG to the corresponding membrane receptor. To determine the cellular targets, PBMC were fractionated into E-rosetting cells (T cells) and non E-rosetting cells (B cells). The suppressive effect was induced by pre-incubation of either T cells or B cells with the GG preparations for 1 h, at 37 degrees C in PWM-induced immunoglobulin (Ig) production. The failure of T cells pretreated with OKT8 monoclonal antibody and complement to induce the suppressive effect suggested that T8 positive T cells are one of the effector cells involved. The activation step of the suppressive effect was prostaglandin E2-independent, and as effector cells contain an Fc receptor which is sensitive to pronase, it was suggested that monocytes were not involved in this activation process. Our observations further suggested that the Ig effects of GG therapy are not limited to antibody transfer, since GG preparations also suppress directly the differentiation of B cells and induce suppressor T cells in in vitro immunoglobulin production stimulated with PWM.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colombatti M., Heumann D., Moretta L. Distribution and properties of Fc receptors for IgG on different leucocyte populations in man. Clin Exp Immunol. 1981 Nov;46(2):453–458. [PMC free article] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Griscelli C. Dysfunctions of pokeweed mitogen-stimulated T and B lymphocyte responses induced by gammaglobulin therapy. J Clin Invest. 1981 Mar;67(3):867–877. doi: 10.1172/JCI110104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A., Durandy A., Griscelli C. Role of prostaglandin E2 in the induction of nonspecific T lymphocyte suppressor activity. J Immunol. 1981 Apr;126(4):1452–1455. [PubMed] [Google Scholar]
- Hoffmann M. K., Kappler J. W. Two distinct mechanisms of immune suppression by antibody. Nature. 1978 Mar 2;272(5648):64–65. doi: 10.1038/272064a0. [DOI] [PubMed] [Google Scholar]
- Milne R. W., Chalon M. P., Vaerman J. P. Suppression of the in vitro immune response by isolated mouse IgG subclasses. Mol Immunol. 1980 Dec;17(12):1599–1602. doi: 10.1016/0161-5890(80)90186-8. [DOI] [PubMed] [Google Scholar]
- Morell A., Schürch B., Ryser D., Hofer F., Skvaril F., Barandun S. In vivo behaviour of gamma globulin preparations. Vox Sang. 1980;38(5):272–283. doi: 10.1111/j.1423-0410.1980.tb02367.x. [DOI] [PubMed] [Google Scholar]
- Moretta L., Webb S. R., Grossi C. E., Lydyard P. M., Cooper M. D. Functional analysis of two human T-cell subpopulations: help and suppression of B-cell responses by T cells bearing receptors for IgM or IgG. J Exp Med. 1977 Jul 1;146(1):184–200. doi: 10.1084/jem.146.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson J. K., McDougal J. S. Aggregated IgG-induced suppression of in vitro antibody responses: definition of the cellular target(s). J Immunol. 1981 Jun;126(6):2382–2389. [PubMed] [Google Scholar]
- Römer J., Morgenthaler J. J., Scherz R., Skvaril F. Characterization of various immunoglobulin preparations for intravenous application. I. Protein composition and antibody content. Vox Sang. 1982 Feb;42(2):62–73. doi: 10.1159/000460850. [DOI] [PubMed] [Google Scholar]
- Samarut C., Cordier G., Revillard J. P. Insoluble immune complexes suppress mitogen-induced proliferation of human T lymphocytes bearing Fc gamma receptors. Cell Immunol. 1979 Jan;42(1):18–27. doi: 10.1016/0008-8749(79)90217-x. [DOI] [PubMed] [Google Scholar]
- Schiff R. I., Rudd C., Johnson R., Buckley R. H. Individualization of gamma globulin dosage in patients with humoral immunodeficiency. Birth Defects Orig Artic Ser. 1983;19(3):209–212. [PubMed] [Google Scholar]
- Sorensen R. U., Polmar S. H. Efficacy and safety of high-dose intravenous immune globulin therapy for antibody deficiency syndromes. Am J Med. 1984 Mar 30;76(3A):83–90. doi: 10.1016/0002-9343(84)90325-5. [DOI] [PubMed] [Google Scholar]
- Uhr J. W., Möller G. Regulatory effect of antibody on the immune response. Adv Immunol. 1968;8:81–127. doi: 10.1016/s0065-2776(08)60465-4. [DOI] [PubMed] [Google Scholar]


