Abstract
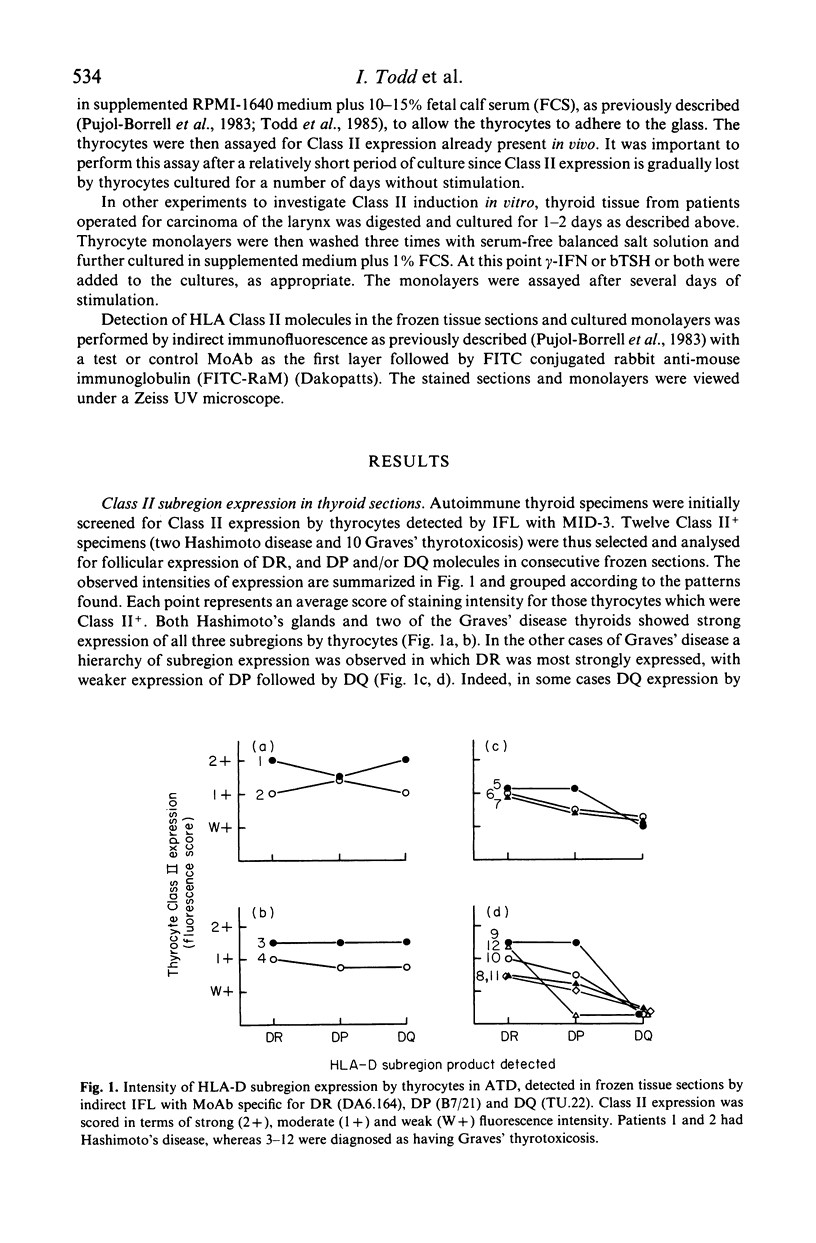
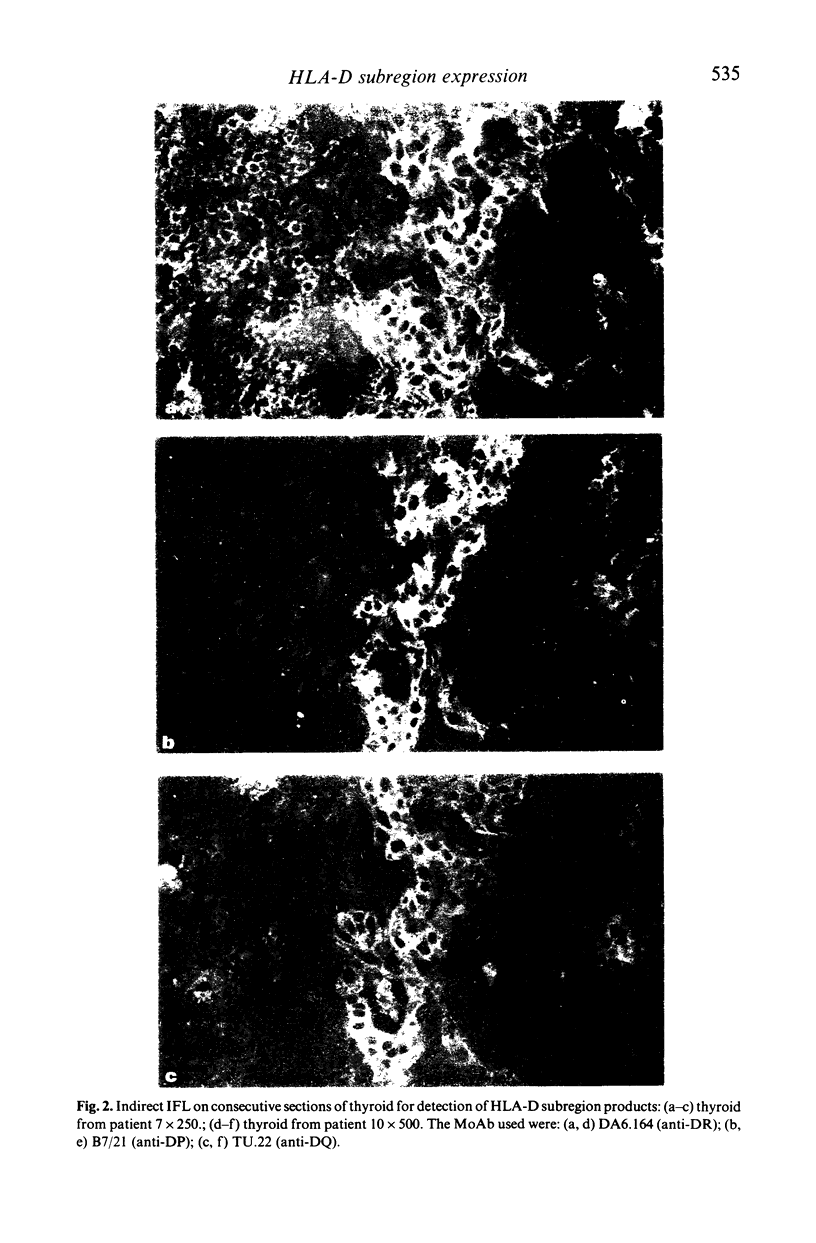
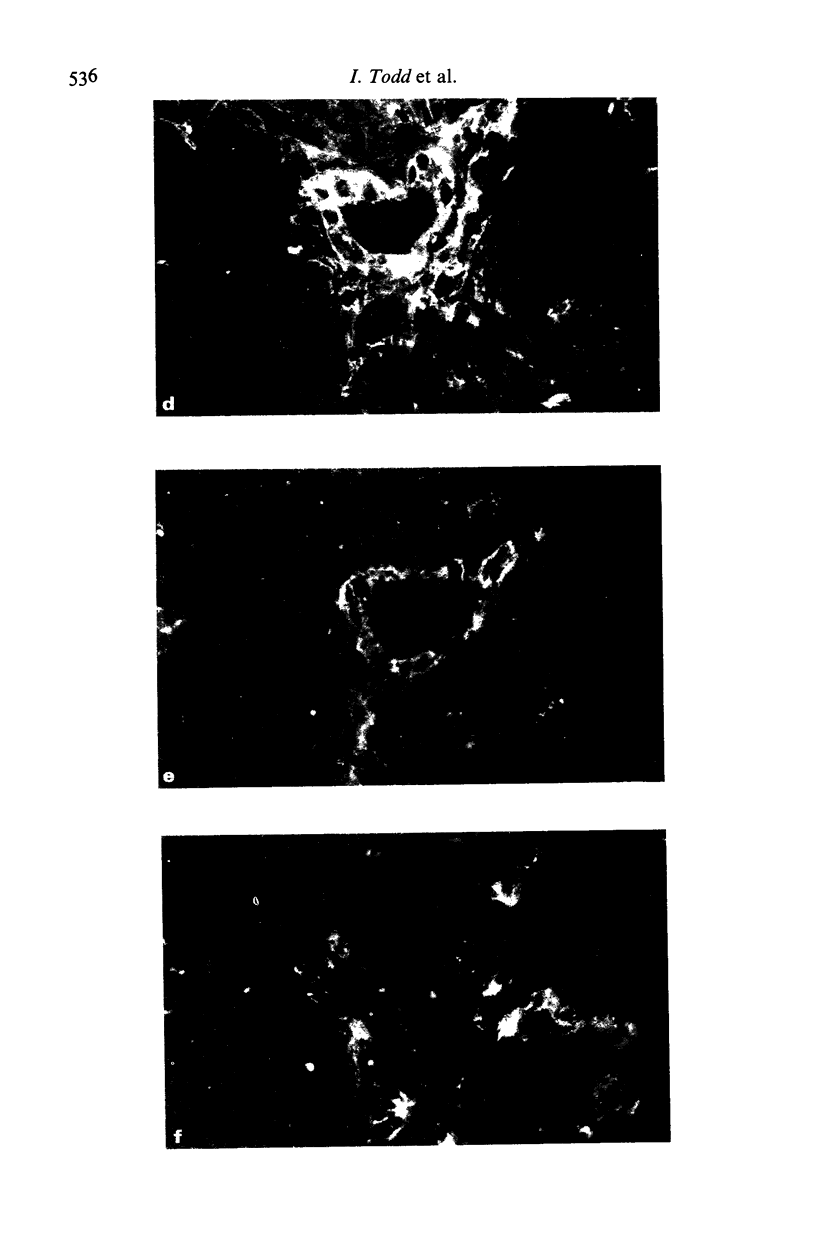
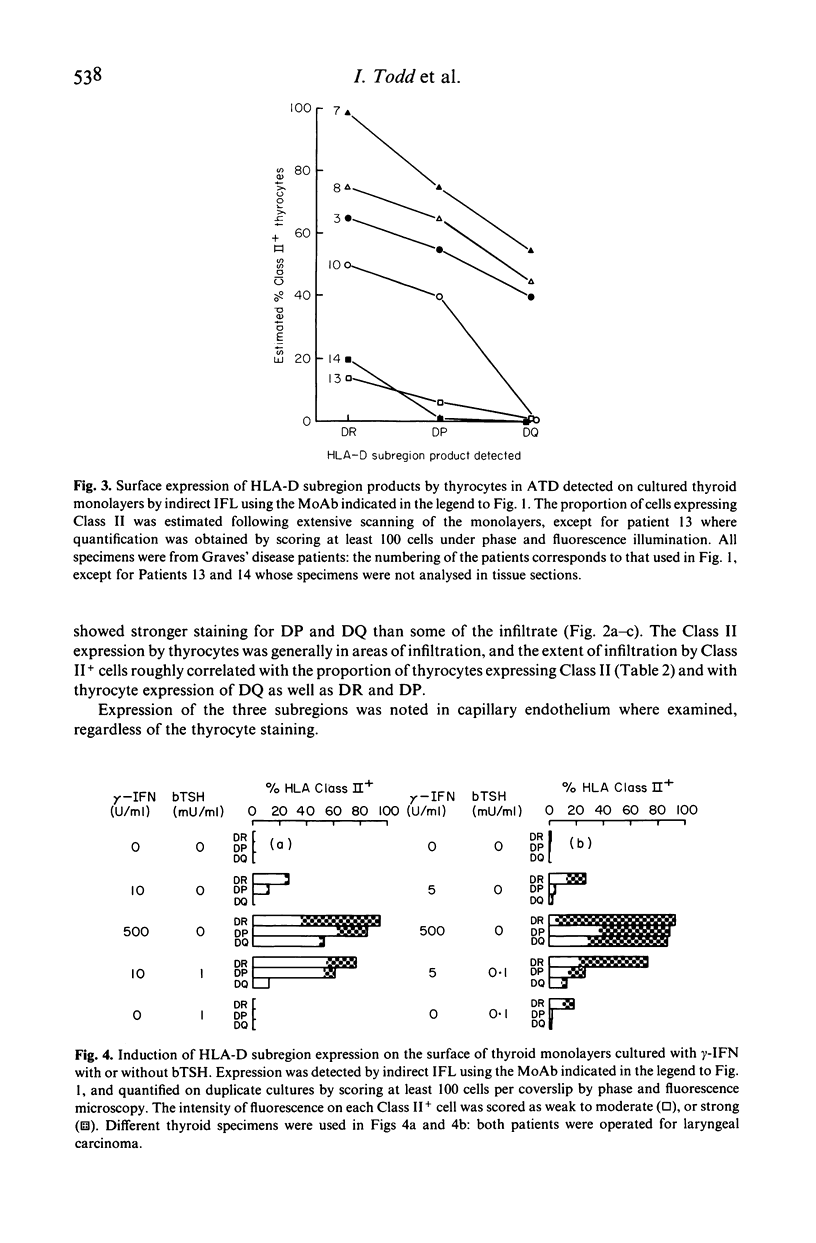
Human thyroid epithelial cells (thyrocytes) express HLA Class II molecules in autoimmune thyroid diseases (ATD). Normal thyrocytes do not express Class II, but can be induced to do so by culture with interferon-gamma (gamma-IFN). We have examined HLA-D subregion expression in sections and monolayers of thyroid by indirect immunofluorescence using appropriate monoclonal antibodies. The results indicate that, in ATD, the incidence and intensity of Class II subregion expression by thyrocytes varies between patients, and follows the pattern DR greater than DP greater than DQ. The same hierarchy is observed in cultured normal thyrocytes treated with gamma-IFN: strong induction of Class II, and of DP and DQ in particular, requires relatively high concentrations of gamma-IFN or additional factors such as thyroid stimulating hormone. These findings suggest that HLA-D subregion expression by thyrocytes in on-going ATD is determined by the levels of disease related factors in the affected tissue.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aichinger G., Fill H., Wick G. In situ immune complexes, lymphocyte subpopulations, and HLA-DR-positive epithelial cells in Hashimoto thyroiditis. Lab Invest. 1985 Feb;52(2):132–140. [PubMed] [Google Scholar]
- Ballardini G., Mirakian R., Bianchi F. B., Pisi E., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigens on bileduct epithelium in primary biliary cirrhosis: relevance to pathogenesis. Lancet. 1984 Nov 3;2(8410):1009–1013. doi: 10.1016/s0140-6736(84)91108-5. [DOI] [PubMed] [Google Scholar]
- Berrih S., Arenzana-Seisdedos F., Cohen S., Devos R., Charron D., Virelizier J. L. Interferon-gamma modulates HLA class II antigen expression on cultured human thymic epithelial cells. J Immunol. 1985 Aug;135(2):1165–1171. [PubMed] [Google Scholar]
- Bottazzo G. F., Dean B. M., McNally J. M., MacKay E. H., Swift P. G., Gamble D. R. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985 Aug 8;313(6):353–360. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- Bottazzo G. F., Todd I., Mirakian R., Belfiore A., Pujol-Borrell R. Organ-specific autoimmunity: a 1986 overview. Immunol Rev. 1986 Dec;94:137–169. doi: 10.1111/j.1600-065X.1986.tb01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel S., Schmidt-Kessen A., Giuffrè L. Recombinant interferon-gamma can induce the expression of HLA-DR and -DC on DR-negative melanoma cells and enhance the expression of HLA-ABC and tumor-associated antigens. Eur J Immunol. 1985 Feb;15(2):118–123. doi: 10.1002/eji.1830150204. [DOI] [PubMed] [Google Scholar]
- Collins T., Korman A. J., Wake C. T., Boss J. M., Kappes D. J., Fiers W., Ault K. A., Gimbrone M. A., Jr, Strominger J. L., Pober J. S. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies T. F. Cocultures of human thyroid monolayer cells and autologous T cells: impact of HLA class II antigen expression. J Clin Endocrinol Metab. 1985 Sep;61(3):418–422. doi: 10.1210/jcem-61-3-418. [DOI] [PubMed] [Google Scholar]
- Fischer A., Sterkers G., Charron D., Durandy A. HLA class II restriction governing cell cooperation between antigen-specific helper T lymphocytes, B lymphocytes and monocytes for in vitro antibody production to influenza virus. Eur J Immunol. 1985 Jun;15(6):620–626. doi: 10.1002/eji.1830150617. [DOI] [PubMed] [Google Scholar]
- Gonwa T. A., Picker L. J., Raff H. V., Goyert S. M., Silver J., Stobo J. D. Antigen-presenting capabilities of human monocytes correlates with their expression of HLA-DS, an Ia determinant distinct from HLA-DR. J Immunol. 1983 Feb;130(2):706–711. [PubMed] [Google Scholar]
- Hanafusa T., Pujol-Borrell R., Chiovato L., Russell R. C., Doniach D., Bottazzo G. F. Aberrant expression of HLA-DR antigen on thyrocytes in Graves' disease: relevance for autoimmunity. Lancet. 1983 Nov 12;2(8359):1111–1115. doi: 10.1016/s0140-6736(83)90628-1. [DOI] [PubMed] [Google Scholar]
- Londei M., Bottazzo G. F., Feldmann M. Human T-cell clones from autoimmune thyroid glands: specific recognition of autologous thyroid cells. Science. 1985 Apr 5;228(4695):85–89. doi: 10.1126/science.3871967. [DOI] [PubMed] [Google Scholar]
- Möst J., Knapp W., Wick G. Class II antigens in Hashimoto thyroiditis. I. Synthesis and expression of HLA-DR and HLA-DQ by thyroid epithelial cells. Clin Immunol Immunopathol. 1986 Nov;41(2):165–174. doi: 10.1016/0090-1229(86)90100-5. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Segatto O., Ferrone S., Tosi R., Corte G. Differential tissue distribution and ontogeny of DC-1 and HLA-DR antigens. Immunogenetics. 1984;19(2):109–116. doi: 10.1007/BF00387853. [DOI] [PubMed] [Google Scholar]
- Rayner D. C., Lydyard P. M., de Assis-Paiva H. J., Bidey S., van der Meide P., Varey A. M., Cooke A. Interferon-mediated enhancement of thyroid major histocompatibility complex antigen expression. A flow cytometric analysis. Scand J Immunol. 1987 Jun;25(6):621–628. doi: 10.1111/j.1365-3083.1987.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Salamero J., Charreire J. Syngeneic sensitization of mouse lymphocytes on monolayers of thyroid epithelial cells. V. The primary syngeneic sensitization is under I-A subregion control. Eur J Immunol. 1983 Nov;13(11):948–951. doi: 10.1002/eji.1830131115. [DOI] [PubMed] [Google Scholar]
- Sone T., Tsukamoto K., Hirayama K., Nishimura Y., Takenouchi T., Aizawa M., Sasazuki T. Two distinct class II molecules encoded by the genes within HLA-DR subregion of HLA-Dw2 and Dw12 can act as stimulating and restriction molecules. J Immunol. 1985 Aug;135(2):1288–1298. [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., Bottazzo G. F., Feldmann M. Interferon-gamma induces HLA-DR expression by thyroid epithelium. Clin Exp Immunol. 1985 Aug;61(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Todd I., Pujol-Borrell R., Hammond L. J., McNally J. M., Feldmann M., Bottazzo G. F. Enhancement of thyrocyte HLA class II expression by thyroid stimulating hormone. Clin Exp Immunol. 1987 Sep;69(3):524–531. [PMC free article] [PubMed] [Google Scholar]
- Warford A., McLachlan S. M., Malcolm A. J., Young E. T., Farndon J. R., Rees Smith B. Characterization of lymphoid cells in the thyroid of patients with Graves' disease. Clin Exp Immunol. 1984 Sep;57(3):626–632. [PMC free article] [PubMed] [Google Scholar]
- Weetman A. P., Volkman D. J., Burman K. D., Gerrard T. L., Fauci A. S. The in vitro regulation of human thyrocyte HLA-DR antigen expression. J Clin Endocrinol Metab. 1985 Nov;61(5):817–824. doi: 10.1210/jcem-61-5-817. [DOI] [PubMed] [Google Scholar]
- Zoppi G., Gasparini R., Mantovanelli F., Gobio-Casali L., Astolfi R., Crovari P. Diet and antibody response to vaccinations in healthy infants. Lancet. 1983 Jul 2;2(8340):11–14. doi: 10.1016/s0140-6736(83)90004-1. [DOI] [PubMed] [Google Scholar]


