Abstract
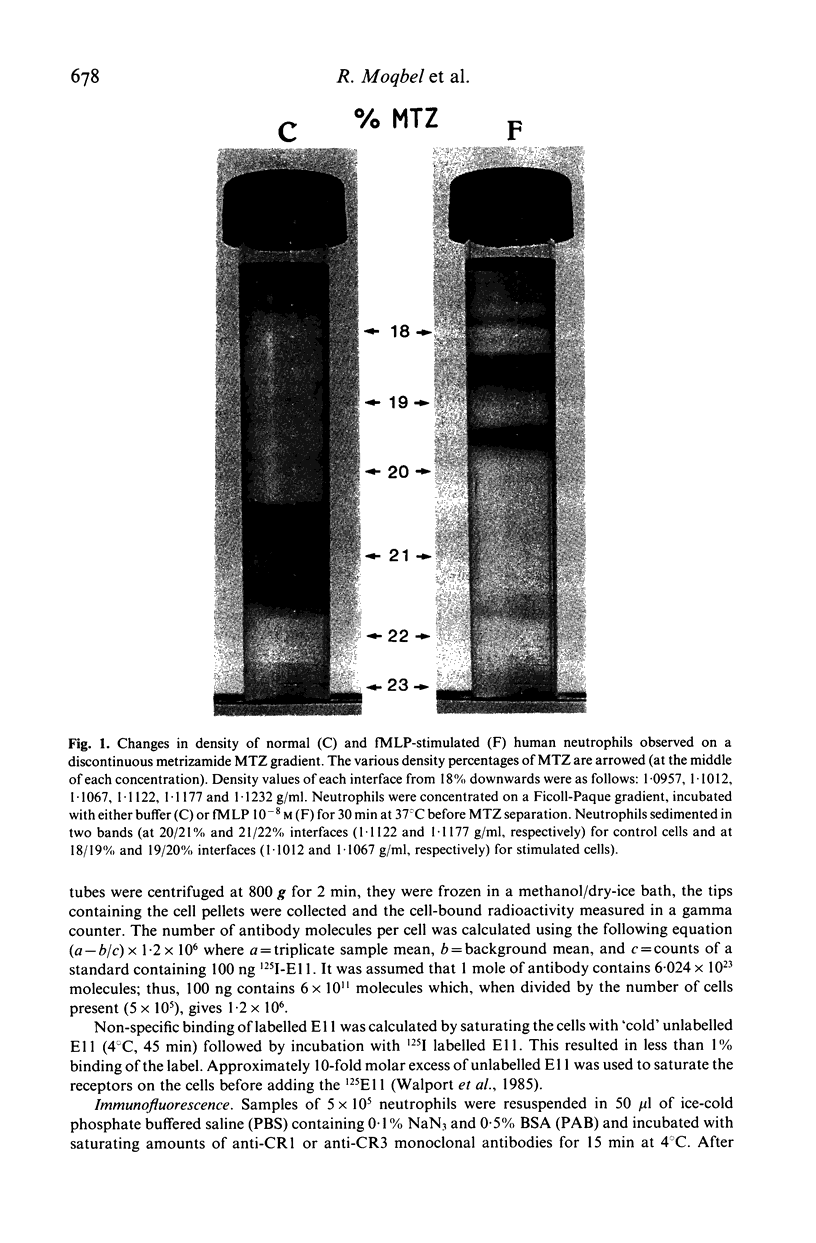
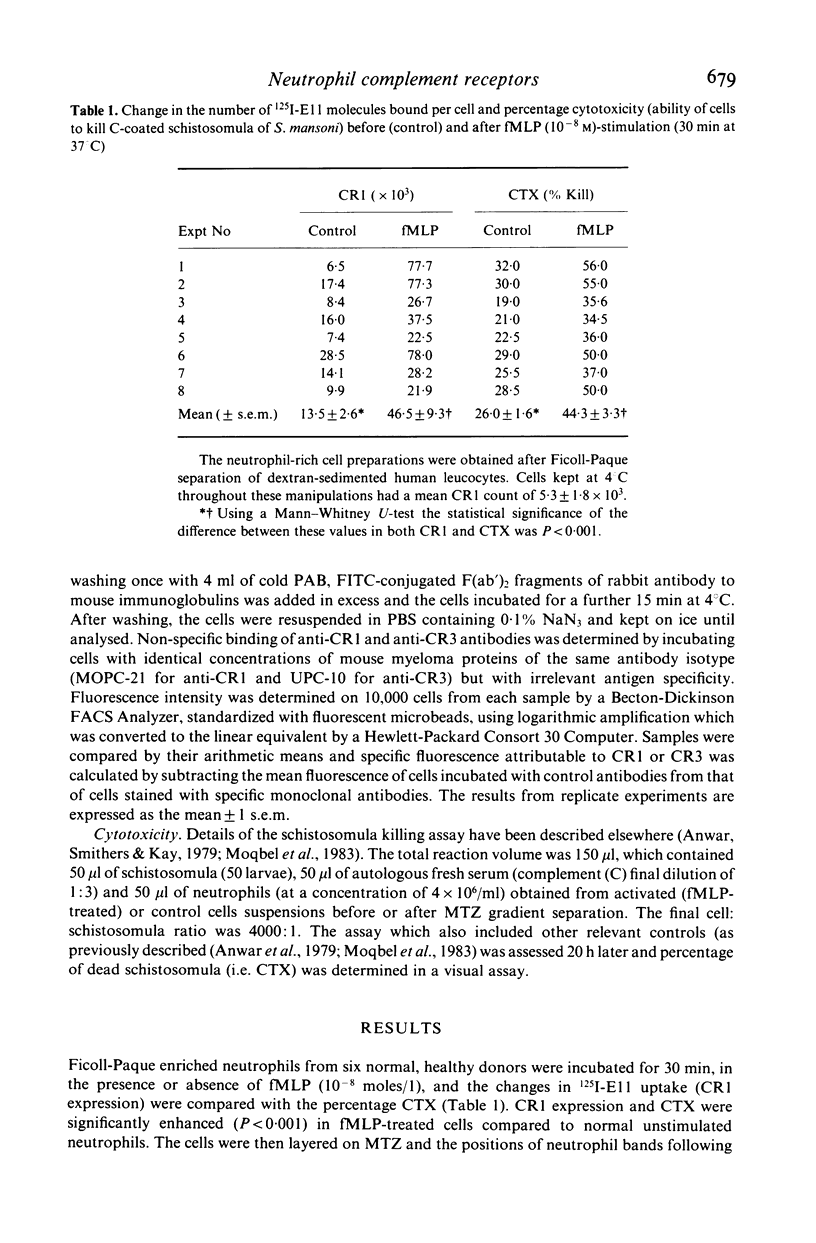
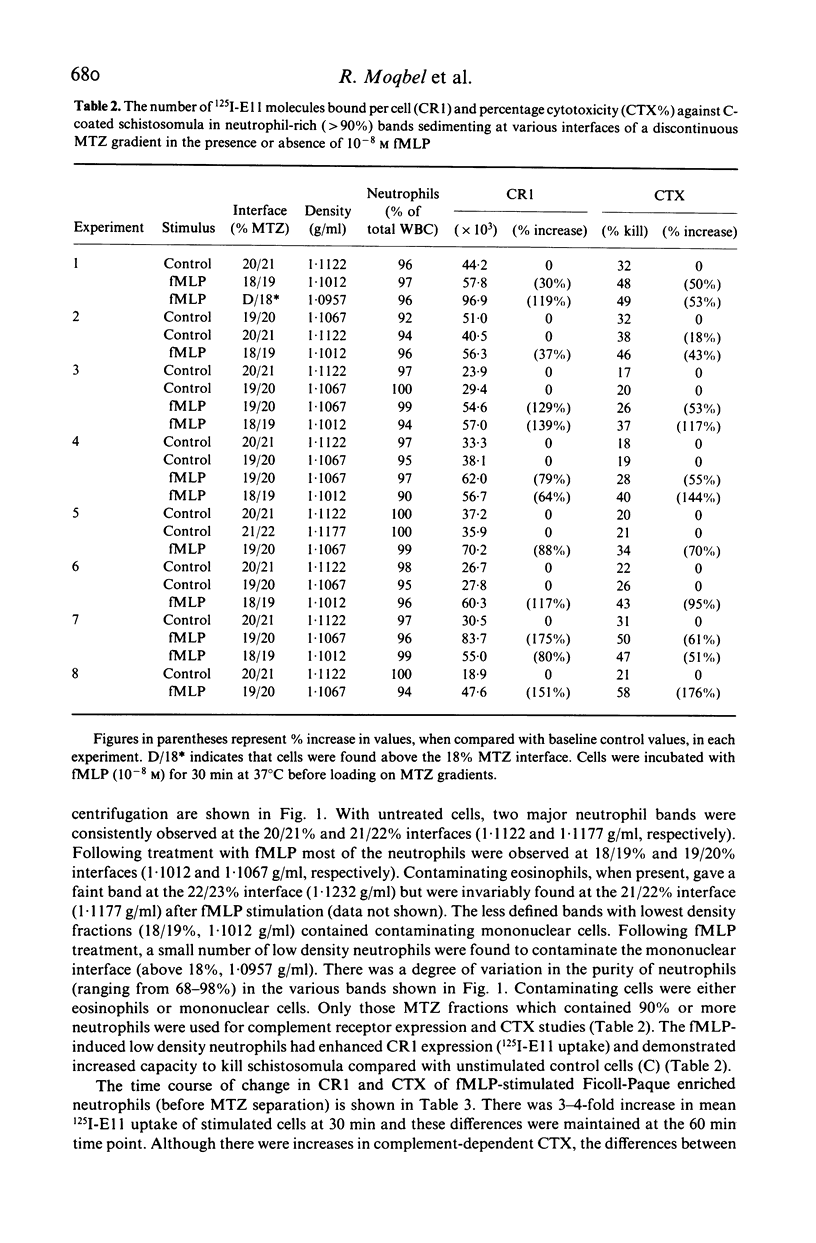
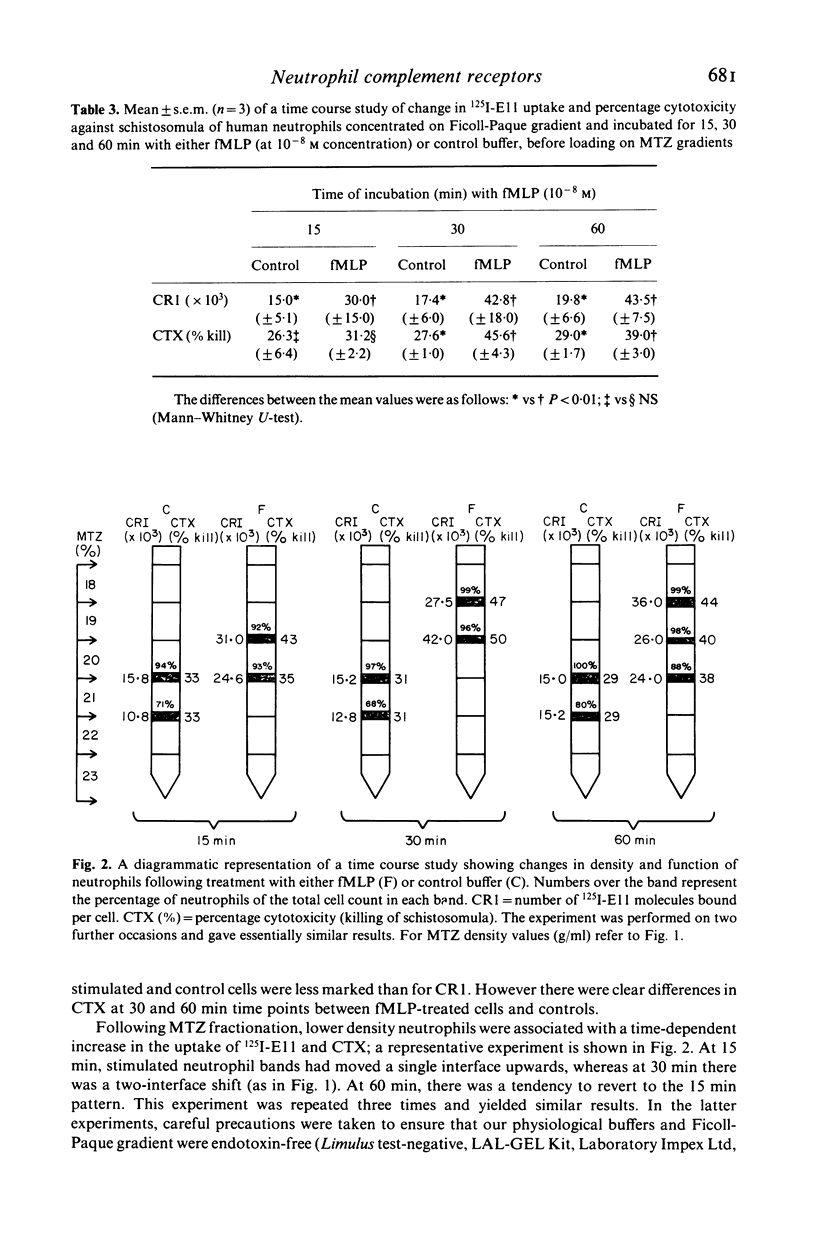
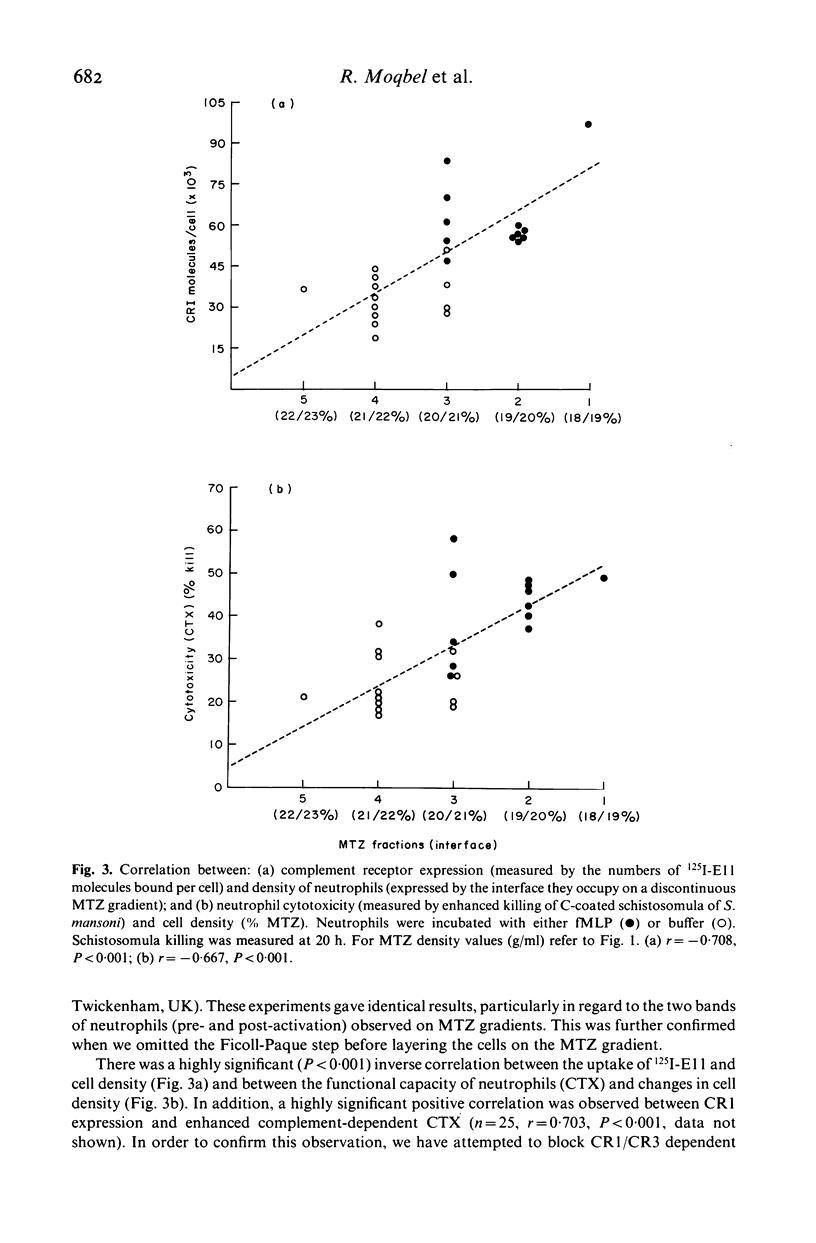
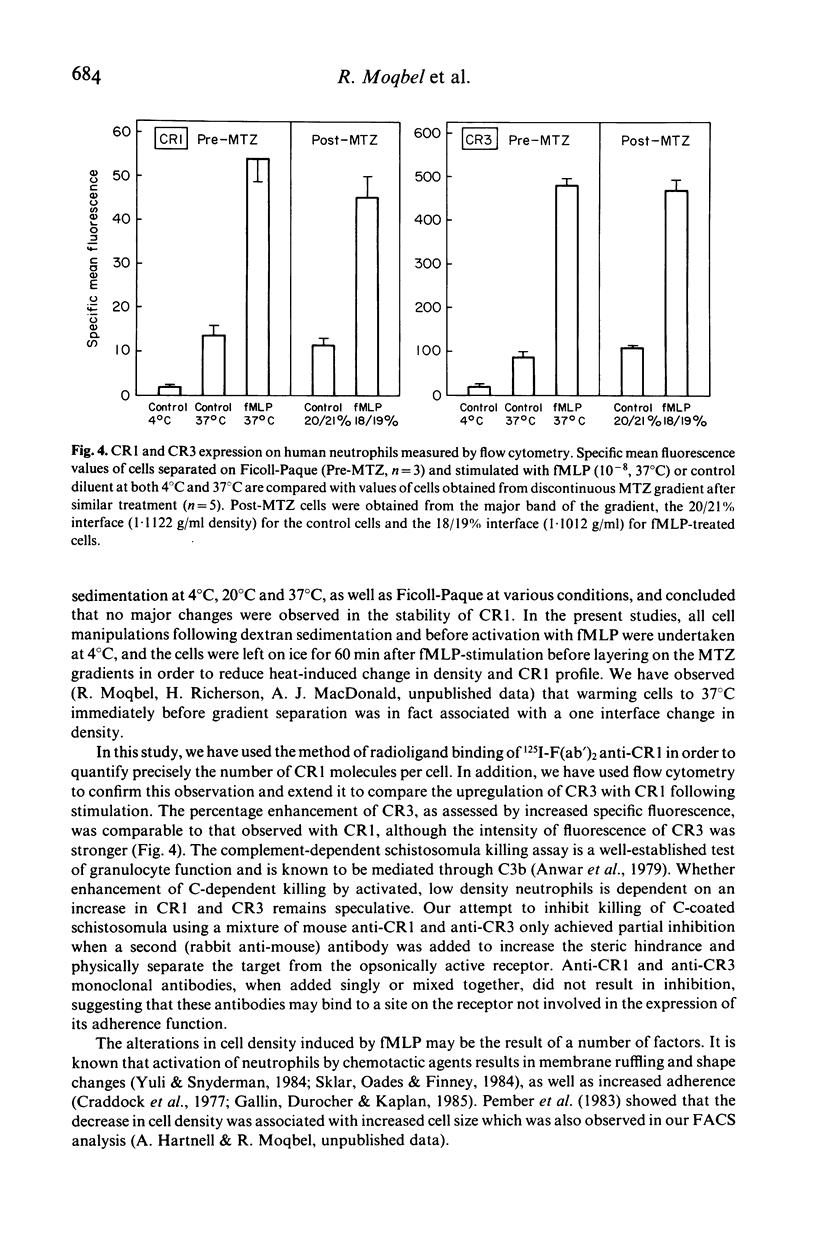
We have studied chemotactic factor-induced 'complement receptor enhancement' to determine whether changes in receptor expression and complement-dependent cytotoxicity were associated with alterations in cell density. Ficoll-Paque separated normal human neutrophils (greater than 90%), when further fractionated on discontinuous metrizamide (MTZ) gradients (18, 19, 20, 21, 22, 23% MTZ), consistently gave two major bands at the 20/21% and 21/22% interfaces. Incubation with the synthetic chemotactic peptide, N-formyl-methionyl-leucyl-phenylalanine (fMLP (10(-8) M)), converted virtually all neutrophils to low density cells sedimenting on MTZ at the 18/19% and 19/20% interfaces. There was a time-dependent change of density after fMLP-stimulation which was maximal at 30 min, with cells reverting towards normal density by 60 min. Control unstimulated cells did not alter their density at any of the time points examined. Activated, low density neutrophils had increased expression of CR1 and CR3 (as shown by flow cytometry and the uptake of 125I-F(ab')2 monoclonal anti-CR1 antibody (E11)). These cells also showed enhanced cytotoxic capacity in vitro for helminthic targets (schistosomula of Schistosoma mansoni) opsonized with autologous complement. There were highly significant correlations between cell density and anti-CR1 uptake (P less than 0.001), and between schistosomular killing and change in density (P less than 0.001). Increased CR1 expression also correlated with enhanced helminthicidal capacity of neutrophils (P less than 0.001). Complement dependent cytotoxicity was partially reduced after treatment of cells with anti-human CR1 and/or CR3 antibodies, but only in the presence of a second antibody. These findings indicate that chemotactic factor-induced complement receptor enhancement of human neutrophils is associated with a decrease in cell density and increased complement-dependent cytotoxicity (CTX).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar A. R., Smithers S. R., Kay A. B. Killing of schistosomula of Schistosoma mansoni coated with antibody and/or complement by human leukocytes in vitro: requirement for complement in preferential killing by eosinophils. J Immunol. 1979 Feb;122(2):628–637. [PubMed] [Google Scholar]
- Berger M., Birx D. L., Wetzler E. M., O'Shea J. J., Brown E. J., Cross A. S. Calcium requirements for increased complement receptor expression during neutrophil activation. J Immunol. 1985 Aug;135(2):1342–1348. [PubMed] [Google Scholar]
- Berger M., O'Shea J., Cross A. S., Folks T. M., Chused T. M., Brown E. J., Frank M. M. Human neutrophils increase expression of C3bi as well as C3b receptors upon activation. J Clin Invest. 1984 Nov;74(5):1566–1571. doi: 10.1172/JCI111572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capron M., Spiegelberg H. L., Prin L., Bennich H., Butterworth A. E., Pierce R. J., Ouaissi M. A., Capron A. Role of IgE receptors in effector function of human eosinophils. J Immunol. 1984 Jan;132(1):462–468. [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone C., Donneli G., Meli D., Rosati F., Sorice F. Human eosinophils and parasitic diseases. II. Characterization of two cell fractions isolated at different densities. Clin Exp Immunol. 1982 Apr;48(1):249–255. [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Collins L. A. Increased expression of C3b receptors on polymorphonuclear leukocytes induced by chemotactic factors and by purification procedures. J Immunol. 1983 Jan;130(1):370–375. [PubMed] [Google Scholar]
- Gallin J. I., Durocher J. R., Kaplan A. P. Interaction of leukocyte chemotactic factors with the cell surface. I. Chemotactic factor-induced changes in human granulocyte surface charge. J Clin Invest. 1975 May;55(5):967–974. doi: 10.1172/JCI108026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Seligmann B. E. Mobilization and adaptation of human neutrophil chemoattractant fMet-Leu-Phe receptors. Fed Proc. 1984 Sep;43(12):2732–2736. [PubMed] [Google Scholar]
- Hogg N., Ross G. D., Jones D. B., Slusarenko M., Walport M. J., Lachmann P. J. Identification of an anti-monocyte monoclonal antibody that is specific for membrane complement receptor type one (CR1). Eur J Immunol. 1984 Mar;14(3):236–243. doi: 10.1002/eji.1830140307. [DOI] [PubMed] [Google Scholar]
- Kay A. B., Glass E. J., Salter D. M. Leucoattractants enhance complement receptors on human phagocytic cells. Clin Exp Immunol. 1979 Nov;38(2):294–299. [PMC free article] [PubMed] [Google Scholar]
- Moqbel R., Sass-Kuhn S. P., Goetzl E. J., Kay A. B. Enhancement of neutrophil- and eosinophil-mediated complement-dependent killing of schistosomula of Schistosoma mansoni in vitro by leukotriene B4. Clin Exp Immunol. 1983 Jun;52(3):519–527. [PMC free article] [PubMed] [Google Scholar]
- Pember S. O., Barnes K. C., Brandt S. J., Kinkade J. M., Jr Density heterogeneity of neutrophilic polymorphonuclear leukocytes: gradient fractionation and relationship to chemotactic stimulation. Blood. 1983 Jun;61(6):1105–1115. [PubMed] [Google Scholar]
- Pember S. O., Kinkade J. M., Jr Differences in myeloperoxidase activity from neutrophilic polymorphonuclear leukocytes of differing density: relationship to selective exocytosis of distinct forms of the enzyme. Blood. 1983 Jun;61(6):1116–1124. [PubMed] [Google Scholar]
- Prin L., Capron M., Tonnel A. B., Bletry O., Capron A. Heterogeneity of human peripheral blood eosinophils: variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int Arch Allergy Appl Immunol. 1983;72(4):336–346. doi: 10.1159/000234893. [DOI] [PubMed] [Google Scholar]
- Richerson H. B., Walsh G. M., Walport M. J., Moqbel R., Kay A. B. Enhancement of human neutrophil complement receptors: a comparison of the rosette technique with the uptake of radio-labelled anti-CR1 monoclonal antibody. Clin Exp Immunol. 1985 Nov;62(2):442–448. [PMC free article] [PubMed] [Google Scholar]
- Roberts R. L., Gallin J. I. Rapid method for isolation of normal human peripheral blood eosinophils on discontinuous Percoll gradients and comparison with neutrophils. Blood. 1985 Feb;65(2):433–440. [PubMed] [Google Scholar]
- Seligmann B., Chused T. M., Gallin J. I. Differential binding of chemoattractant peptide to subpopulations of human neutrophils. J Immunol. 1984 Nov;133(5):2641–2646. [PubMed] [Google Scholar]
- Shaw R. J., Walsh G. M., Cromwell O., Moqbel R., Spry C. J., Kay A. B. Activated human eosinophils generate SRS-A leukotrienes following IgG-dependent stimulation. Nature. 1985 Jul 11;316(6024):150–152. doi: 10.1038/316150a0. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Oades Z. G., Finney D. A. Neutrophil degranulation detected by right angle light scattering: spectroscopic methods suitable for simultaneous analyses of degranulation or shape change, elastase release, and cell aggregation. J Immunol. 1984 Sep;133(3):1483–1487. [PubMed] [Google Scholar]
- Tai P. C., Spry C. J. Studies on blood eosinophils. I. Patients with a transient eosinophilia. Clin Exp Immunol. 1976 Jun;24(3):415–422. [PMC free article] [PubMed] [Google Scholar]
- Vadas M. A., David J. R., Butterworth A., Pisani N. T., Siongok T. A. A new method for the purification of human eosinophils and neutrophils, and a comparison of the ability of these cells to damage schistosomula of Schistosoma mansoni. J Immunol. 1979 Apr;122(4):1228–1236. [PubMed] [Google Scholar]
- Walport M. J., Ross G. D., Mackworth-Young C., Watson J. V., Hogg N., Lachmann P. J. Family studies of erythrocyte complement receptor type 1 levels: reduced levels in patients with SLE are acquired, not inherited. Clin Exp Immunol. 1985 Mar;59(3):547–554. [PMC free article] [PubMed] [Google Scholar]



