Abstract
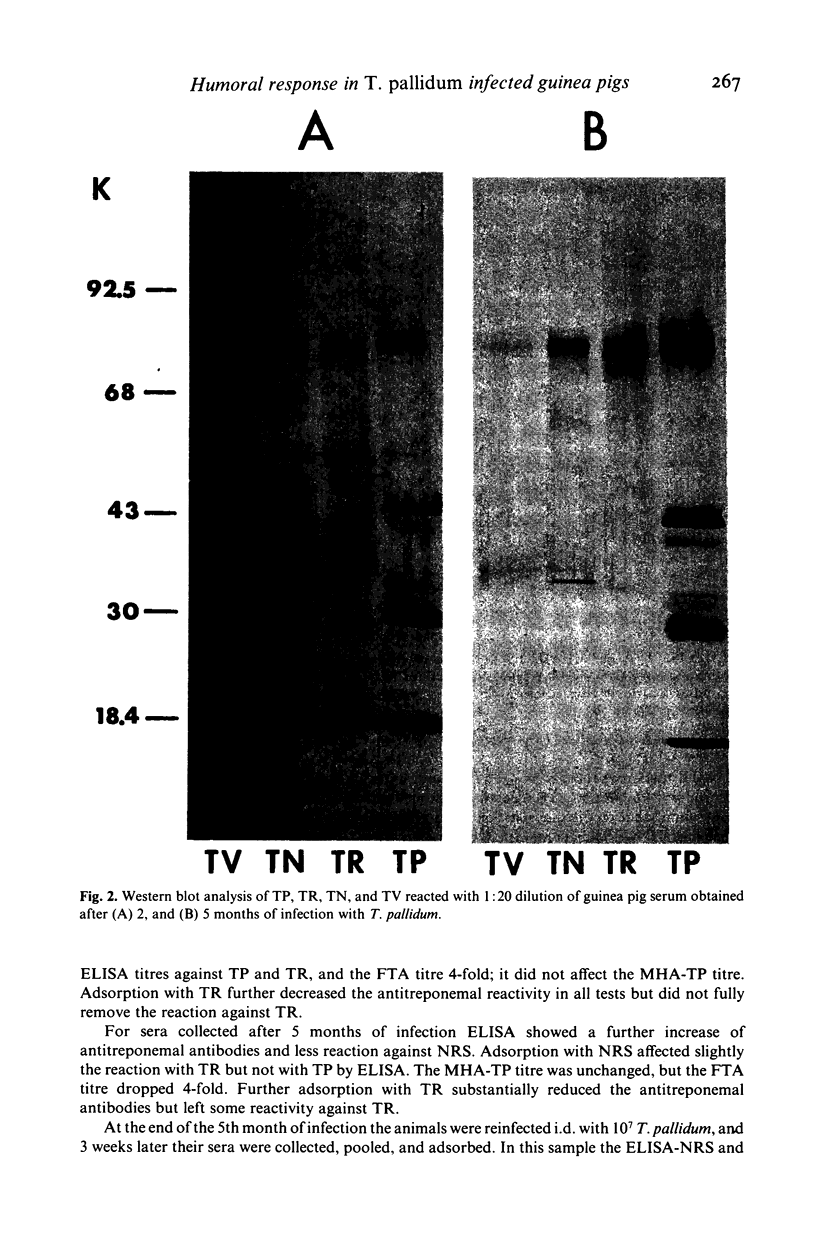
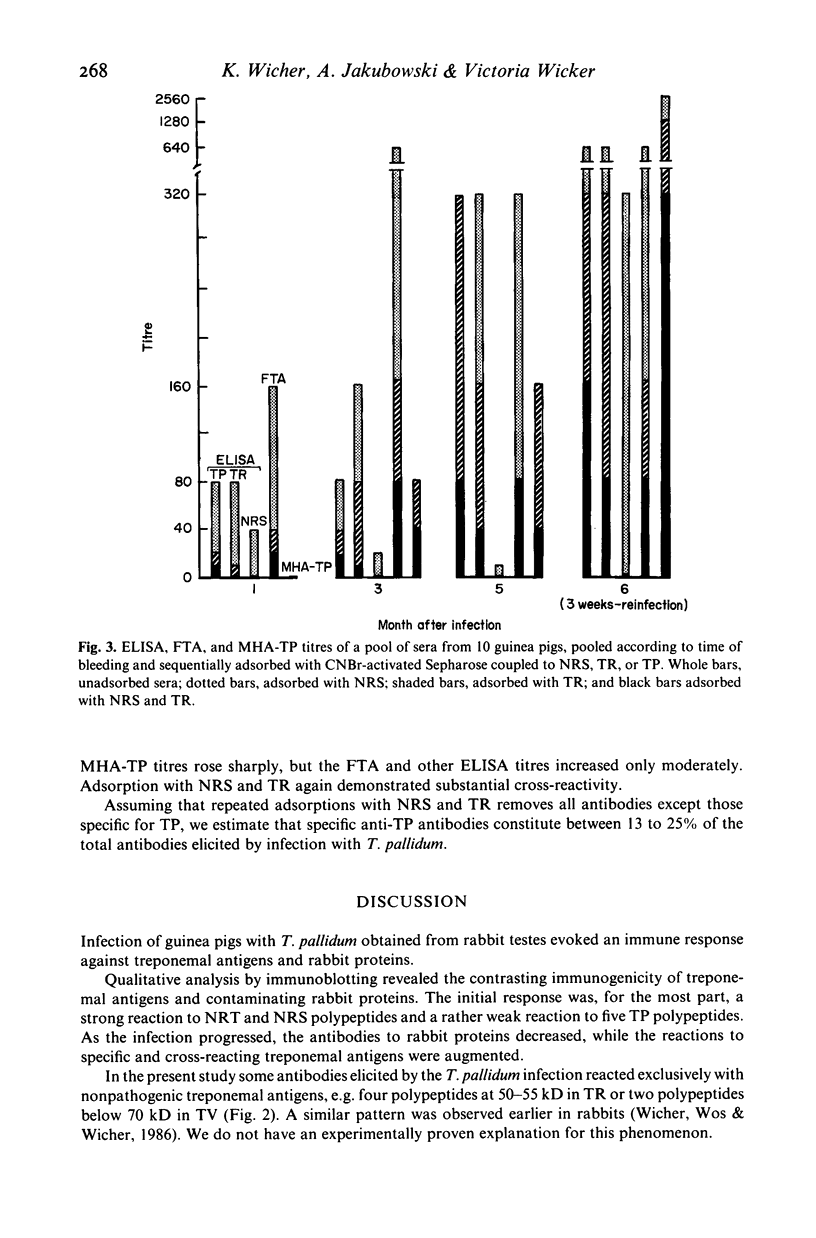
Young male inbred strain 2 guinea pigs were infected intradermally with 8 X 10(7) Treponema pallidum extracted from a rabbit orchitis, and 5 months later reinfected with 10(7) T. pallidum. Ninety percent of the animals developed symptomatic lesions after initial infection but none on challenge. Immunoblotting of sera obtained at intervals after infection or reinfection showed antibodies against T. pallidum antigen (TP), nonpathogenic treponemes--T. phagedenis biotype Reiter (TR), T. refringens strain Noguchi (TN), and T. vincentii (TV)--as well as normal rabbit serum (NRS) and normal rabbit testes extract (NRT). Antibodies reacting with TP were detected as early as 17 days (five polypeptides) and steadily rose (at 3 months 17 polypeptides were seen). Cross-reacting antibodies to TR, TN, TV, or rabbit proteins decreased within 3 to 5 months. After reinfection, the antibodies to NRS increased more sharply than the anti-treponemal antibodies. Adsorption with TR and NRS of sera obtained after infection or reinfection produced a reduction of antibodies to TP by 75-87%.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughn R. E., McNeely M. C., Jorizzo J. L., Musher D. M. Characterization of the antigenic determinants and host components in immune complexes from patients with secondary syphilis. J Immunol. 1986 Feb 15;136(4):1406–1414. [PubMed] [Google Scholar]
- Baughn R. E., Musher D. M. Isolation and preliminary characterization of circulating immune complexes from rabbits with experimental syphilis. Infect Immun. 1983 Nov;42(2):579–584. doi: 10.1128/iai.42.2.579-584.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughn R. E., Tung K. S., Musher D. M. Detection of circulating immune complexes in the sera of rabbits with experimental syphilis: possible role in immunoregulation. Infect Immun. 1980 Aug;29(2):575–582. doi: 10.1128/iai.29.2.575-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casavant C. H., Wicher V., Wicher K. Host response to Treponema pallidum infection. III. Demonstration of autoantibodies to heart in sera from infected rabbits. Int Arch Allergy Appl Immunol. 1978;56(2):171–178. [PubMed] [Google Scholar]
- Fitzgerald T. J., Repesh L. A., Blanco D. R., Miller J. N. Attachment of Treponema pallidum to fibronectin, laminin, collagen IV, and collagen I, and blockage of attachment by immune rabbit IgG. Br J Vener Dis. 1984 Dec;60(6):357–363. doi: 10.1136/sti.60.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lukehart S. A., Baker-Zander S. A., Gubish E. R., Jr Identification of Treponema pallidum antigens: comparison with a nonpathogenic treponeme. J Immunol. 1982 Aug;129(2):833–838. [PubMed] [Google Scholar]
- MILGROM F., WITEBSKY E. Autoantibodies and autoimmune diseases. JAMA. 1962 Aug 25;181:706–716. [PubMed] [Google Scholar]
- Pavia C. S., Niederbuhl C. J. Experimental infection of inbred guinea pigs with Treponema pallidum: development of lesions and formation of antibodies. Genitourin Med. 1985 Apr;61(2):75–81. doi: 10.1136/sti.61.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce C. S., Wicher K., Nakeeb S. Experimental syphilis: guinea pig model. Br J Vener Dis. 1983 Jun;59(3):157–168. doi: 10.1136/sti.59.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS T. E., HIDALGO R. J., DIMOPOULLOS G. T. IMMUNOLOGY AND SEROLOGY OF ANAPLASMA MARGINALE. I. FRACTIONATION OF THE COMPLEMENT-FIXING ANTIGEN. J Bacteriol. 1964 Jul;88:81–86. doi: 10.1128/jb.88.1.81-86.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strugnell R. A., Williams W. F., Raines G., Pedersen J. S., Drummond L. P., Toh B. H., Faine S. Autoantibodies to creatine kinase in rabbits infected with Treponema pallidum. J Immunol. 1986 Jan;136(2):667–671. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WICHER K., JAKUBOWSKI A. EFFECT OF CORTISONE ON THE COURSE OF EXPERIMENTAL SYPHILIS IN THE GUINEA-PIG. I. EFFECT OF PREVIOUSLY-ADMINISTERED CORTISONE ON GUINEA-PIGS INFECTED WITH TREPONEMA PALLIDUM INTRADERMALLY, INTRATESTICULARLY, AND INTRAVENOUSLY. Br J Vener Dis. 1964 Sep;40:213–216. doi: 10.1136/sti.40.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wicher K., Wicher V., Gruhn R. F. Differences in susceptibility to infection with Treponema pallidum (Nichols) between five strains of guinea pig. Genitourin Med. 1985 Feb;61(1):21–26. doi: 10.1136/sti.61.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher K., Wicher V., Nakeeb S. M., Dubiski S. Studies of rabbit testes infected with Treponema pallidum. I Immunopathology. Br J Vener Dis. 1983 Dec;59(6):349–358. doi: 10.1136/sti.59.6.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicher K., Wicher V., Wang M. C. Cellular and humoral immune response to guinea pig infected with Treponema pallidum. Int Arch Allergy Appl Immunol. 1976;51(3):284–297. doi: 10.1159/000231603. [DOI] [PubMed] [Google Scholar]
- Wicher V., Wicher K. Lymphocyte transformation in inbred guinea pigs infected with Treponema pallidum nichols. Int Arch Allergy Appl Immunol. 1985;76(3):266–269. doi: 10.1159/000233703. [DOI] [PubMed] [Google Scholar]
- Wos S. M., Wicher K. Antigenic evidence for host origin of exudative fluids in lesions of Treponema pallidum-infected rabbits. Infect Immun. 1985 Jan;47(1):228–233. doi: 10.1128/iai.47.1.228-233.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wos S. M., Wicher K. Extensive cross reactivity between Treponema pallidum and cultivable treponemes demonstrated by sequential immunoadsorption. Int Arch Allergy Appl Immunol. 1986;79(3):282–285. doi: 10.1159/000233987. [DOI] [PubMed] [Google Scholar]




