Abstract
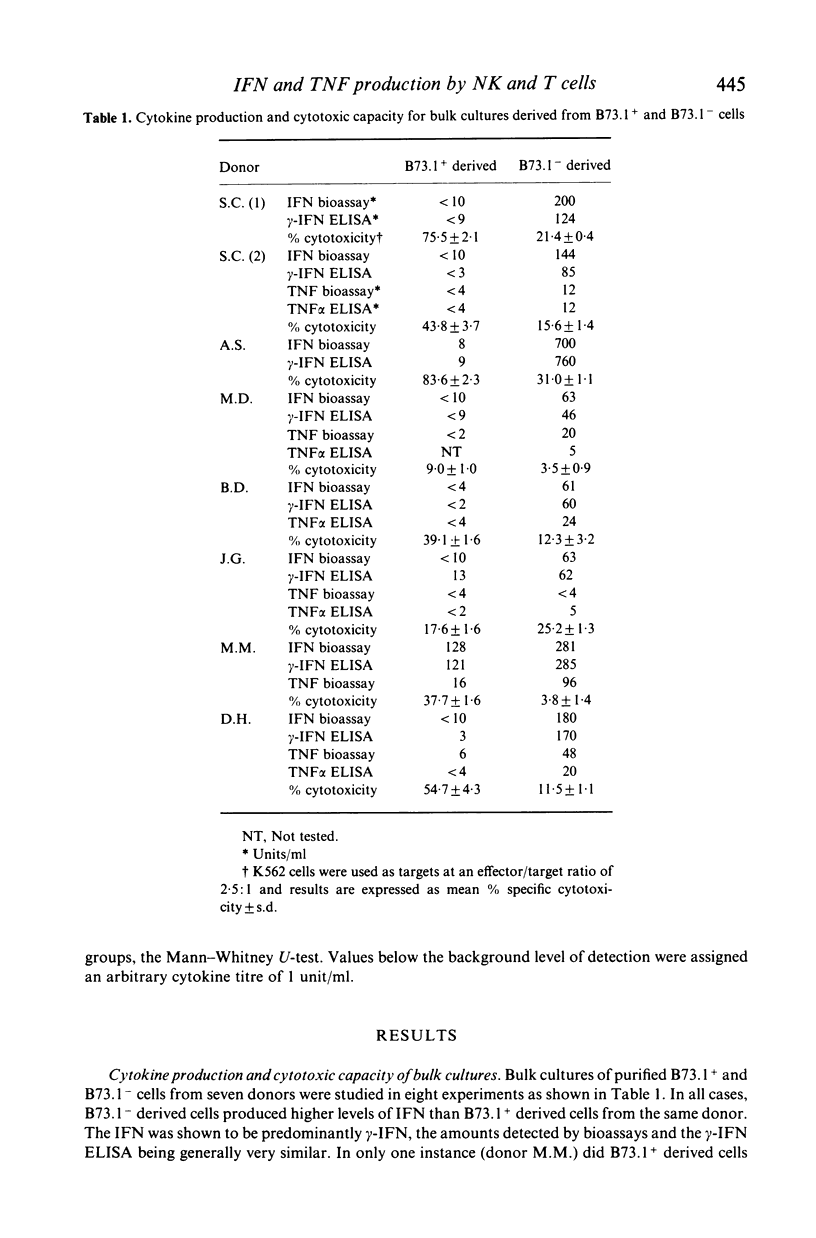
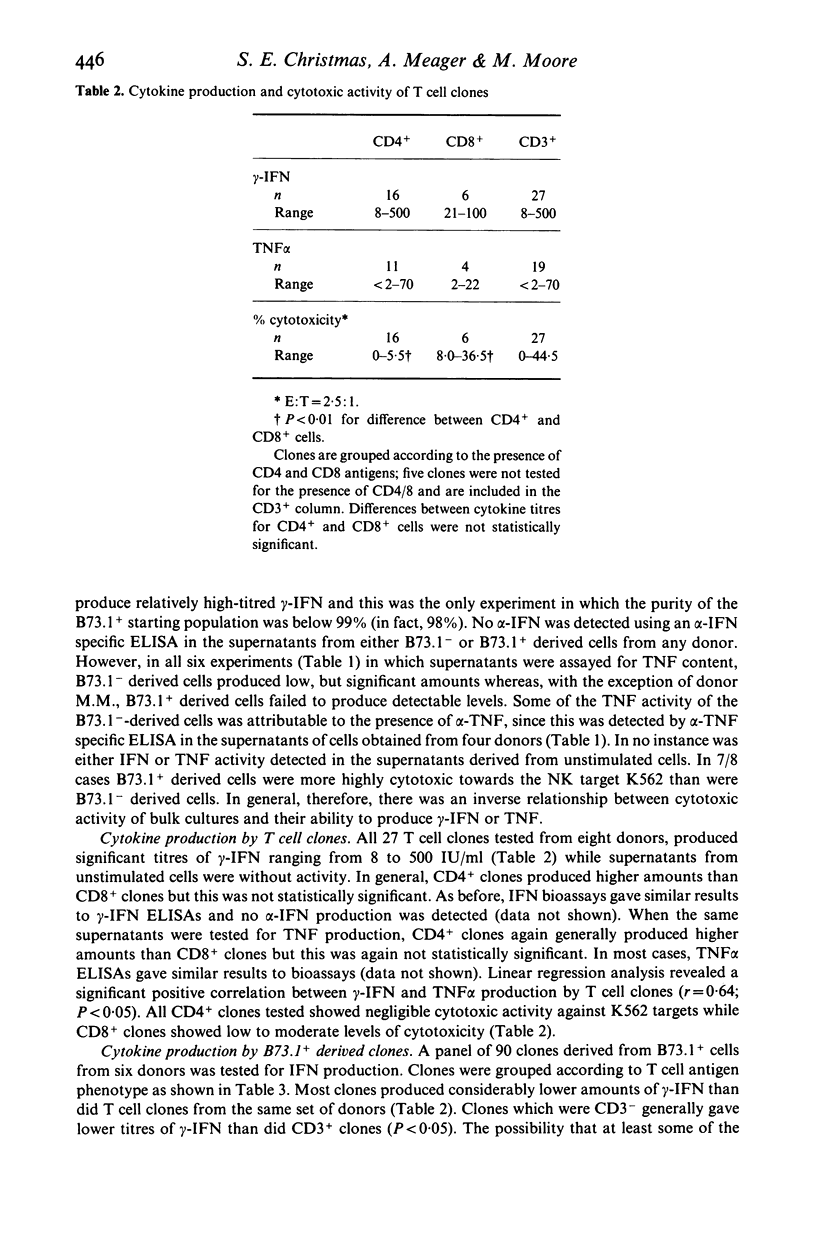
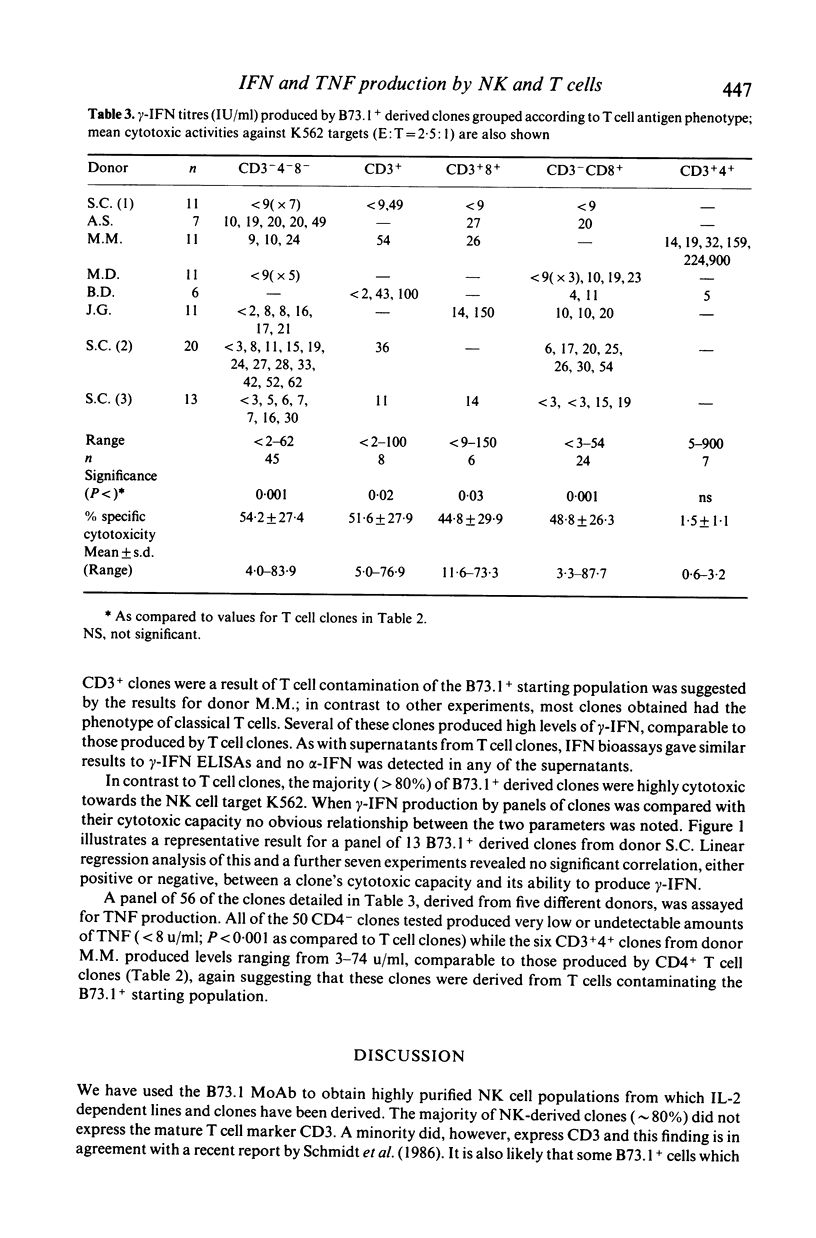
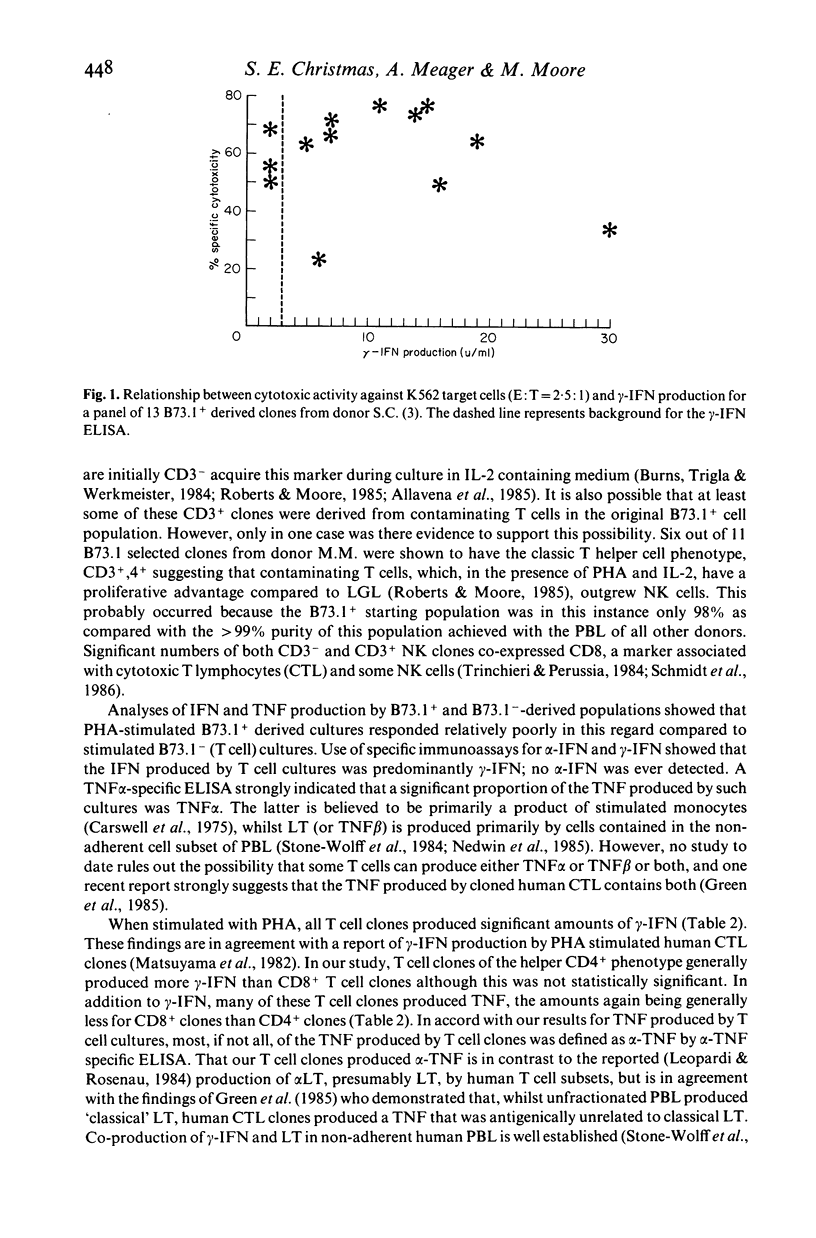
Cell lines and clones, derived from natural killer (NK) cell-enriched (B73.1+) peripheral blood lymphocytes (PBL) from several human donors, that expressed distinct surface phenotypes and were cytolytically active against K562 target cells were tested for their capacity to produce interferon (IFN) and tumour necrosis factor (TNF), IFN and TNF were measured firstly in biological assays and secondly in specific immunoassays for alpha-IFN, gamma-IFN and tumour necrosis factor (TNF alpha). It was found that the majority of NK-derived lines and clones were highly cytotoxic towards K562, but generally produced relatively low or undetectable levels of gamma-IFN and TNF alpha following stimulation with phytohaemagglutinin. No alpha-IFN was detected in supernatants from these cells. In comparison, cell lines and clones, derived from T lymphocyte (B73.1-) enriched PBL from the same donors were poorly cytotoxic towards K562, but generally produced higher levels of gamma-IFN and TNF than NK-derived cells. Thus, neither gamma-IFN nor TNF production were shown to correlate well with the capacity of NK-derived or T cell clones to effect cytotoxic action towards K562 in vitro. These results suggest that the co-production of gamma-IFN and TNF is not indicative of cytotoxic potential.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allavena P., Scala G., Djeu J. Y., Procopio A. D., Oppenheim J. J., Herberman R. B., Ortaldo J. R. Production of multiple cytokines by clones of human large granular lymphocytes. Cancer Immunol Immunother. 1985;19(2):121–126. doi: 10.1007/BF00199719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Degliantoni G., Murphy M., Kobayashi M., Francis M. K., Perussia B., Trinchieri G. Natural killer (NK) cell-derived hematopoietic colony-inhibiting activity and NK cytotoxic factor. Relationship with tumor necrosis factor and synergism with immune interferon. J Exp Med. 1985 Nov 1;162(5):1512–1530. doi: 10.1084/jem.162.5.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Stocks N., Zoon K., Stanton G. J., Timonen T., Herberman R. B. Positive self regulation of cytotoxicity in human natural killer cells by production of interferon upon exposure to influenza and herpes viruses. J Exp Med. 1982 Oct 1;156(4):1222–1234. doi: 10.1084/jem.156.4.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Meager A. Immune interferon produced to high levels by antigenic stimulation of human lymphocytes with influenza virus. J Exp Med. 1981 Nov 1;154(5):1279–1289. doi: 10.1084/jem.154.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A. K., Spriggs A. I., Mason D. Y. Immunocytochemical staining of T and B lymphocytes in serous effusions. J Clin Pathol. 1985 Jun;38(6):608–612. doi: 10.1136/jcp.38.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. M., Stern M. L., Haviland D. L., Mills B. J., Ware C. F. Cytotoxic lymphokines produced by cloned human cytotoxic T lymphocytes. I. Cytotoxins produced by antigen-specific and natural killer-like CTL are dissimilar to classical lymphotoxins. J Immunol. 1985 Dec;135(6):4034–4043. [PubMed] [Google Scholar]
- Grönberg A., Kiessling R., Masucci G., Guevara L. A., Eriksson E., Klein G. Gamma-interferon (IFN-gamma) produced during effector and target interactions renders target cells less susceptible to NK-cell-mediated lysis. Int J Cancer. 1983 Nov 15;32(5):609–616. doi: 10.1002/ijc.2910320515. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Kasahara T., Djeu J. Y., Dougherty S. F., Oppenheim J. J. Capacity of human large granular lymphocytes (LGL) to produce multiple lymphokines: interleukin 2, interferon, and colony stimulating factor. J Immunol. 1983 Nov;131(5):2379–2385. [PubMed] [Google Scholar]
- Kasahara T., Hooks J. J., Dougherty S. F., Oppenheim J. J. Interleukin 2-mediated immune interferon (IFN-gamma) production by human T cells and T cell subsets. J Immunol. 1983 Apr;130(4):1784–1789. [PubMed] [Google Scholar]
- Leist T., Titmas R., Parti S., Meager A. Antibodies to synthetic polypeptides corresponding to hydrophilic regions of human interferon gamma. Mol Immunol. 1985 Aug;22(8):929–936. doi: 10.1016/0161-5890(85)90079-3. [DOI] [PubMed] [Google Scholar]
- Leopardi E., Rosenau W. Production of alpha-lymphotoxin by human T-cell subsets. Cell Immunol. 1984 Jan;83(1):73–82. doi: 10.1016/0008-8749(84)90226-0. [DOI] [PubMed] [Google Scholar]
- Matsuyama M., Sugamura K., Kawade Y., Hinuma Y. Production of immune interferon by human cytotoxic T cell clones. J Immunol. 1982 Aug;129(2):450–451. [PubMed] [Google Scholar]
- Meager A., Parti S., Barwick S., Spragg J., O'Hagan K. Detection of hybridomas secreting monoclonal antibodies to human gamma interferon using a rapid screening technique and specificity of certain monoclonal antibodies to gamma interferon. J Interferon Res. 1984 Fall;4(4):619–625. doi: 10.1089/jir.1984.4.619. [DOI] [PubMed] [Google Scholar]
- Moore M., White W. J., Potter M. R. Modulation of target cell susceptibility to human natural killer cells by interferon. Int J Cancer. 1980 May 15;25(5):565–572. doi: 10.1002/ijc.2910250504. [DOI] [PubMed] [Google Scholar]
- Nedwin G. E., Svedersky L. P., Bringman T. S., Palladino M. A., Jr, Goeddel D. V. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J Immunol. 1985 Oct;135(4):2492–2497. [PubMed] [Google Scholar]
- Nocera A., Melioli G., Merli A., Santoro F., Zicca A. In vitro production of different interferon types by cloned human NK cells. Clin Exp Immunol. 1985 May;60(2):274–284. [PMC free article] [PubMed] [Google Scholar]
- O'Malley J. A., Nussbaum-Blumenson A., Sheedy D., Grossmayer B. J., Ozer H. Identification of the T cell subset that produces human gamma interferon. J Immunol. 1982 Jun;128(6):2522–2526. [PubMed] [Google Scholar]
- Ortaldo J. R., Mason A. T., Gerard J. P., Henderson L. E., Farrar W., Hopkins R. F., 3rd, Herberman R. B., Rabin H. Effects of natural and recombinant IL 2 on regulation of IFN gamma production and natural killer activity: lack of involvement of the Tac antigen for these immunoregulatory effects. J Immunol. 1984 Aug;133(2):779–783. [PubMed] [Google Scholar]
- Palacios R., Martinez-Maza O., De Ley M. Production of human immune interferon (Hu IFN-gamma) studied at the single cell level. Origin, evidence for spontaneous secretion and effect of cyclosporin A. Eur J Immunol. 1983 Mar;13(3):221–225. doi: 10.1002/eji.1830130308. [DOI] [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Roberts K., Moore M. A clonal analysis of human peripheral blood lymphocytes displaying natural killer-like activity. Eur J Immunol. 1985 May;15(5):448–456. doi: 10.1002/eji.1830150507. [DOI] [PubMed] [Google Scholar]
- Schmid D. S., Powell M. B., Mahoney K. A., Ruddle N. H. A comparison of lysis mediated by Lyt 2+ TNP-specific cytotoxic-T-lymphocyte (CTL) lines with that mediated by rapidly internalized lymphotoxin-containing supernatant fluids: evidence for a role of soluble mediators in CTL-mediated killing. Cell Immunol. 1985 Jun;93(1):68–82. doi: 10.1016/0008-8749(85)90389-2. [DOI] [PubMed] [Google Scholar]
- Schmidt R. E., Murray C., Daley J. F., Schlossman S. F., Ritz J. A subset of natural killer cells in peripheral blood displays a mature T cell phenotype. J Exp Med. 1986 Jul 1;164(1):351–356. doi: 10.1084/jem.164.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone-Wolff D. S., Yip Y. K., Kelker H. C., Le J., Henriksen-Destefano D., Rubin B. Y., Rinderknecht E., Aggarwal B. B., Vilcek J. Interrelationships of human interferon-gamma with lymphotoxin and monocyte cytotoxin. J Exp Med. 1984 Mar 1;159(3):828–843. doi: 10.1084/jem.159.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedersky L. P., Nedwin G. E., Goeddel D. V., Palladino M. A., Jr Interferon-gamma enhances induction of lymphotoxin in recombinant interleukin 2-stimulated peripheral blood mononuclear cells. J Immunol. 1985 Mar;134(3):1604–1608. [PubMed] [Google Scholar]
- Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984 Oct 1;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Perussia B. Human natural killer cells: biologic and pathologic aspects. Lab Invest. 1984 May;50(5):489–513. [PubMed] [Google Scholar]
- Wilkinson M., Morris A. The E receptor regulates interferon-gamma production: four-receptor model for human lymphocyte activation. Eur J Immunol. 1984 Aug;14(8):708–713. doi: 10.1002/eji.1830140807. [DOI] [PubMed] [Google Scholar]
- Wright S. C., Bonavida B. Studies on the mechanism of natural killer (NK) cell-mediated cytotoxicity (CMC). I. Release of cytotoxic factors specific for NK-sensitive target cells (NKCF) during co-culture of NK effector cells with NK target cells. J Immunol. 1982 Jul;129(1):433–439. [PubMed] [Google Scholar]


