Abstract
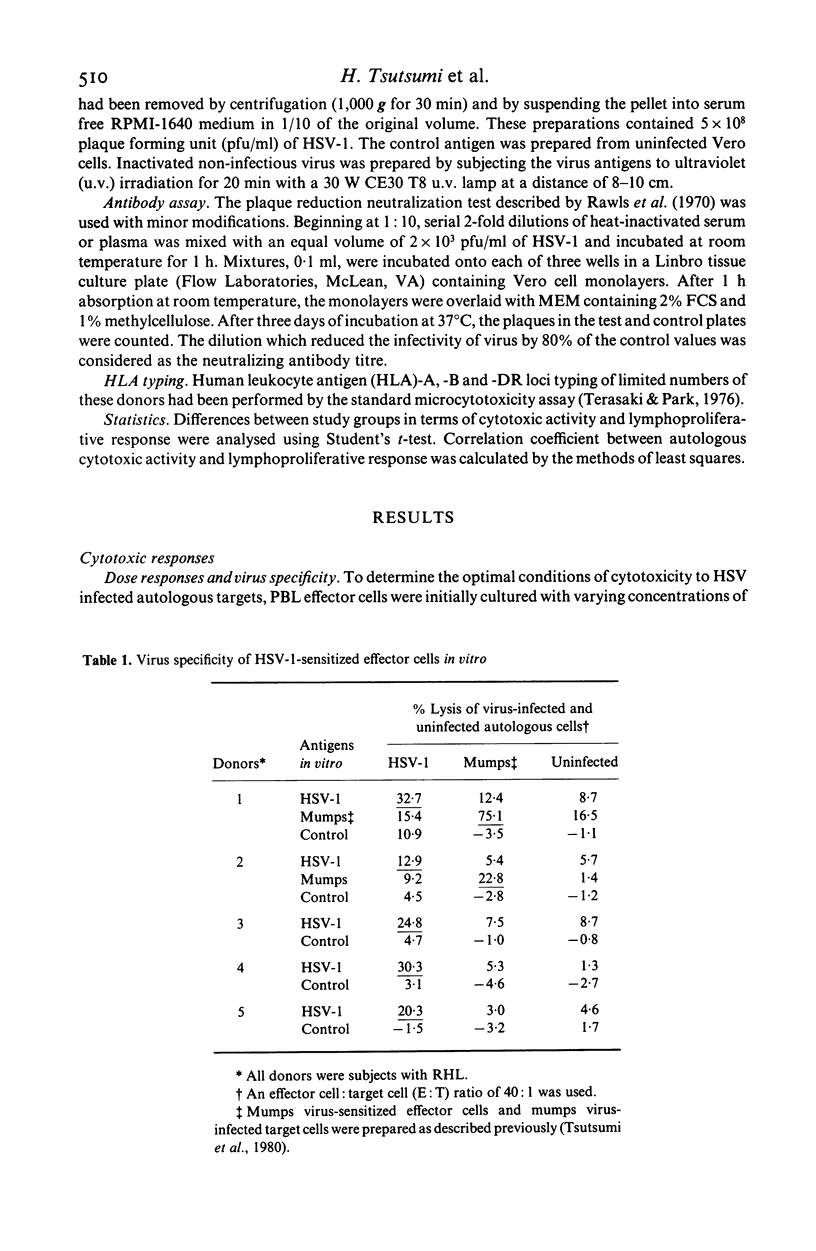
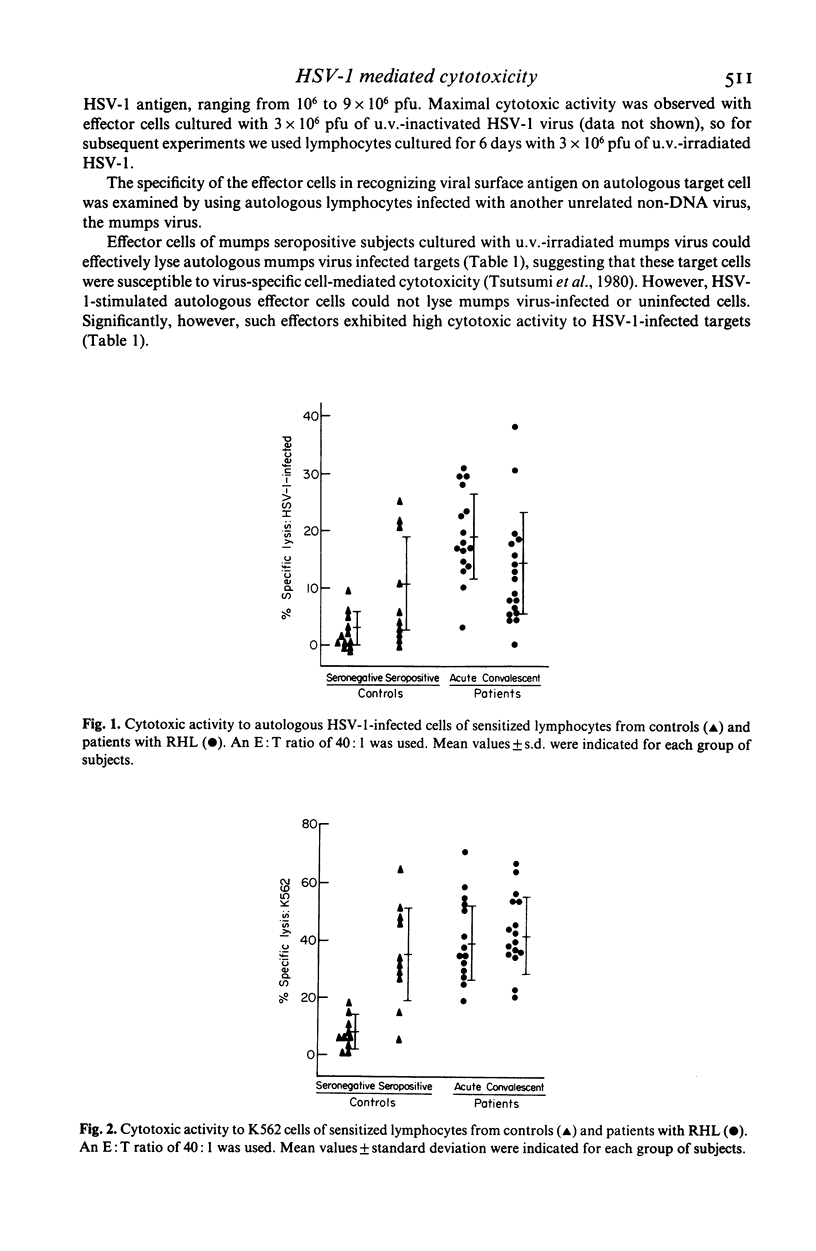
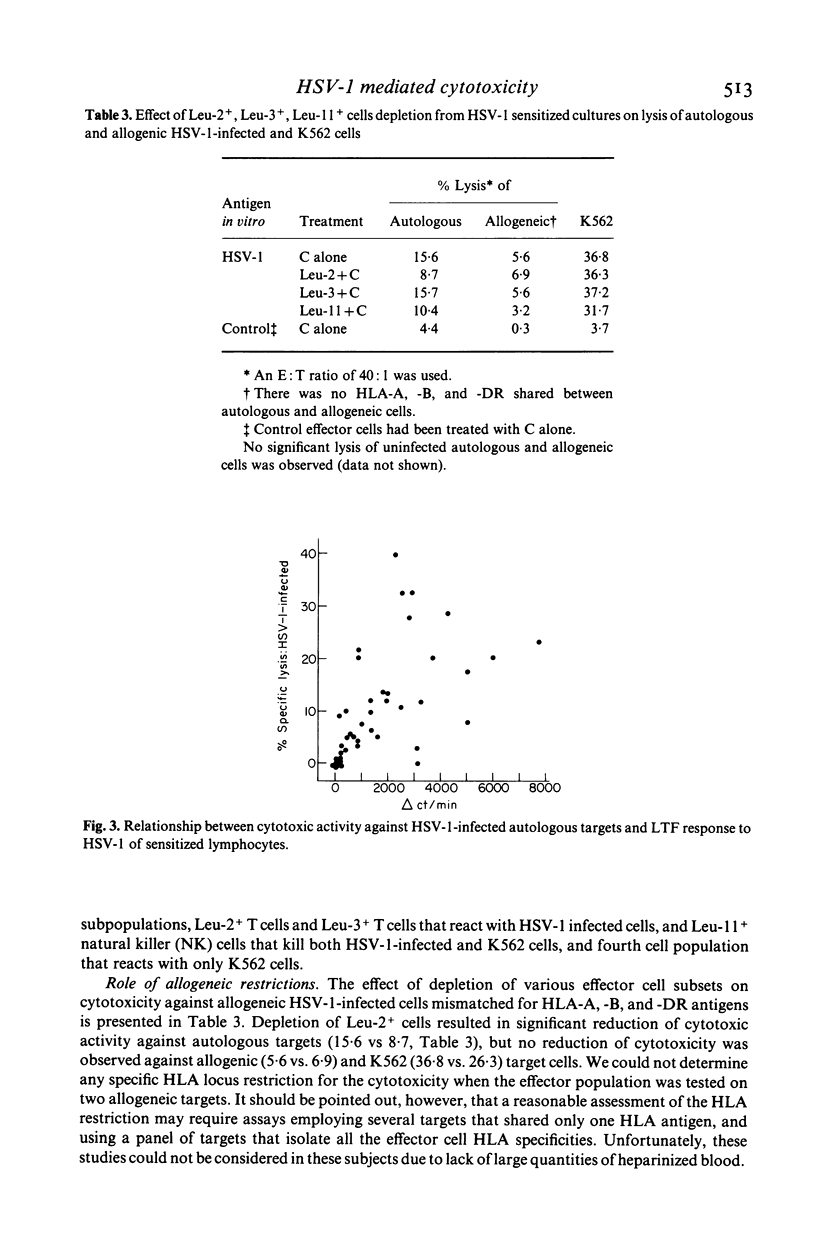
Groups of subjects during acute (0-3 days) and convalescent (2-3 weeks) phase of recurrent herpes labialis (RHL), and other subjects seropositive or seronegative for herpes simplex virus type 1 (HSV-1) antibody without any history of RHL, were tested for the appearance of cell-mediated cytotoxic responses by stimulating peripheral blood leukocytes (PBL) in vitro with ultraviolet-inactivated HSV-1 antigen, using the release of radiolabelled chromium (51Cr) from HSV-1-infected autologous, or allogeneic lymphocytes and K562 erythroleukemia cell line as nonspecific targets. Development of HSV specific cytotoxic response using autologous targets was essentially limited to subjects with RHL and in HSV antibody seropositive control subjects. Peak activity was observed during the acute phase of the disease, compared to the activity in the convalescent phase in seropositive subjects with RHL, and was preceded by high lymphoproliferative response to HSV. Higher cytotoxic responses against K562 cells were also observed in RHL subjects compared to the controls. Depletion of Leu-2+, Leu-3+ or Leu-11 effector lymphocytes from HSV-1-stimulated PBL cultures by treatment with complement and appropriate monoclonal antibodies resulted in significant reduction of cytotoxicity to HSV-1-infected autologous cells. However, cytotoxicity to K562 cells was reduced only after depletion of Leu-11+ cells. Low levels of allogeneic restriction were observed for cytotoxicity to HSV-1-infected targets. These observations suggest selective activation of virus specific Leu-2+ and Leu-3+ T cell subsets as well as natural killer cell mediated cytotoxic mechanisms during the active phase of recurrences of herpes simplex virus infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Byrne J. A., Schreiber R., Patterson S., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus and in vitro properties. J Virol. 1985 Feb;53(2):552–560. doi: 10.1128/jvi.53.2.552-560.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUDDINGH G. J., SCHRUM D. I., LANIER J. C., GUIDRY D. J. Studies of the natural history of herpes simplex infections. Pediatrics. 1953 Jun;11(6):595–610. [PubMed] [Google Scholar]
- Cunningham A. L., Merigan T. C. Leu-3+ T cells produce gamma-interferon in patients with recurrent herpes labialis. J Immunol. 1984 Jan;132(1):197–202. [PubMed] [Google Scholar]
- Cunningham A. L., Merigan T. C. gamma Interferon production appears to predict time of recurrence of herpes labialis. J Immunol. 1983 May;130(5):2397–2400. [PubMed] [Google Scholar]
- Lopez C., O'Reilly R. J. Cell-mediated immune responses in recurrent herpesvirus infections. I. Lymphocyte proliferation assay. J Immunol. 1977 Mar;118(3):895–902. [PubMed] [Google Scholar]
- Lucas C. J., Biddison W. E., Nelson D. L., Shaw S. Killing of measles virus-infected cells by human cytotoxic T cells. Infect Immun. 1982 Oct;38(1):226–232. doi: 10.1128/iai.38.1.226-232.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S. A., Herrmann E. C., Jr, Winkelmann R. K. Herpes simplex infections in hematologic malignancies. Am J Med. 1972 Jan;52(1):102–114. doi: 10.1016/0002-9343(72)90012-5. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Oda H., Mori R., Taniguchi T. Mechanism of acquired resistance to herpes simplex virus infection as studied in nude mice. J Gen Virol. 1979 Sep;44(3):715–723. doi: 10.1099/0022-1317-44-3-715. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Roizman B. Infection with herpes-simplex viruses 1 and 2. 1. N Engl J Med. 1973 Sep 27;289(13):667–674. doi: 10.1056/NEJM197309272891305. [DOI] [PubMed] [Google Scholar]
- O'Reilly R. J., Chibbaro A., Anger E., Lopez C. Cell-mediated immune responses in patients with recurrent Herpes Simplex infections. II. Infection-associated deficiency of lymphokine production in patients with recurrent herpes labialis or herpes progenitalis. J Immunol. 1977 Mar;118(3):1095–1102. [PubMed] [Google Scholar]
- Pien F. D., Smith T. F., Anderson C. F., Webel M. L., Taswell H. F. Herpesviruses in renal transplant patients. Transplantation. 1973 Nov;16(5):489–495. doi: 10.1097/00007890-197311000-00014. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Rasmussen L. E., Pollard R. B., Arvin A., Merigan T. C. Cellular immunity and herpesvirus infections in cardiac-transplant patients. N Engl J Med. 1977 Jun 16;296(24):1372–1377. doi: 10.1056/NEJM197706162962402. [DOI] [PubMed] [Google Scholar]
- Rawls W. E., Iwamoto K., Adam E., Melnick J. L. Measurement of antibodies to herpesvirus types 1 and 2 in human sera. J Immunol. 1970 Mar;104(3):599–606. [PubMed] [Google Scholar]
- Sethi K. K., Omata Y., Schneweis K. E. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol. 1983 Feb;64(Pt 2):443–447. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Stroehmann I., Brandis H. Human T-cell cultures from virus-sensitized donors can mediate virus-specific and HLA-restricted cell lysis. Nature. 1980 Aug 14;286(5774):718–720. doi: 10.1038/286718a0. [DOI] [PubMed] [Google Scholar]
- Shaw S., Nelson D. L., Shearer G. M. Human cytotoxic response in vitro to trinitrophenyl-modified autologous cells. I. T cell recognition of TNP in association with widely shared antigens. J Immunol. 1978 Jul;121(1):281–289. [PubMed] [Google Scholar]
- Shillitoe E. J., Wilton J. M., Lehner T. Sequential changes in cell-mediated immune responses to herpes simplex virus after recurrent herpetic infection in humans. Infect Immun. 1977 Oct;18(1):130–137. doi: 10.1128/iai.18.1.130-137.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr S. E., Karatela S. A., Shore S. L., Duffey A., Nahmias A. J. Stimulation of human lymphocytes by Herpes simplex virus antigens. Infect Immun. 1975 Jan;11(1):109–112. doi: 10.1128/iai.11.1.109-112.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi H., Chiba Y., Abo W., Chiba S., Nakao T. T-cell-mediated cytotoxic response to mumps virus in humans. Infect Immun. 1980 Oct;30(1):129–134. doi: 10.1128/iai.30.1.129-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M., Ivanyi L., Lehner T. Cell-mediated immunity in Herpesvirus hominis infections. Br Med J. 1972 Mar 18;1(5802):723–726. doi: 10.1136/bmj.1.5802.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. II. Bifunctional clones with cytotoxic and virus-induced proliferative activities exhibit herpes simplex virus type 1 and 2 specific or type common reactivities. J Immunol. 1984 Nov;133(5):2736–2742. [PubMed] [Google Scholar]
- Zarling J. M., Dierckins M. S., Sevenich E. A., Clouse K. A. Stimulation with autologous lymphoblastoid cell lines: lysis of Epstein-Barr virus-positive and -negative cell lines by two phenotypically distinguishable effector cell populations. J Immunol. 1981 Nov;127(5):2118–2123. [PubMed] [Google Scholar]


