Abstract
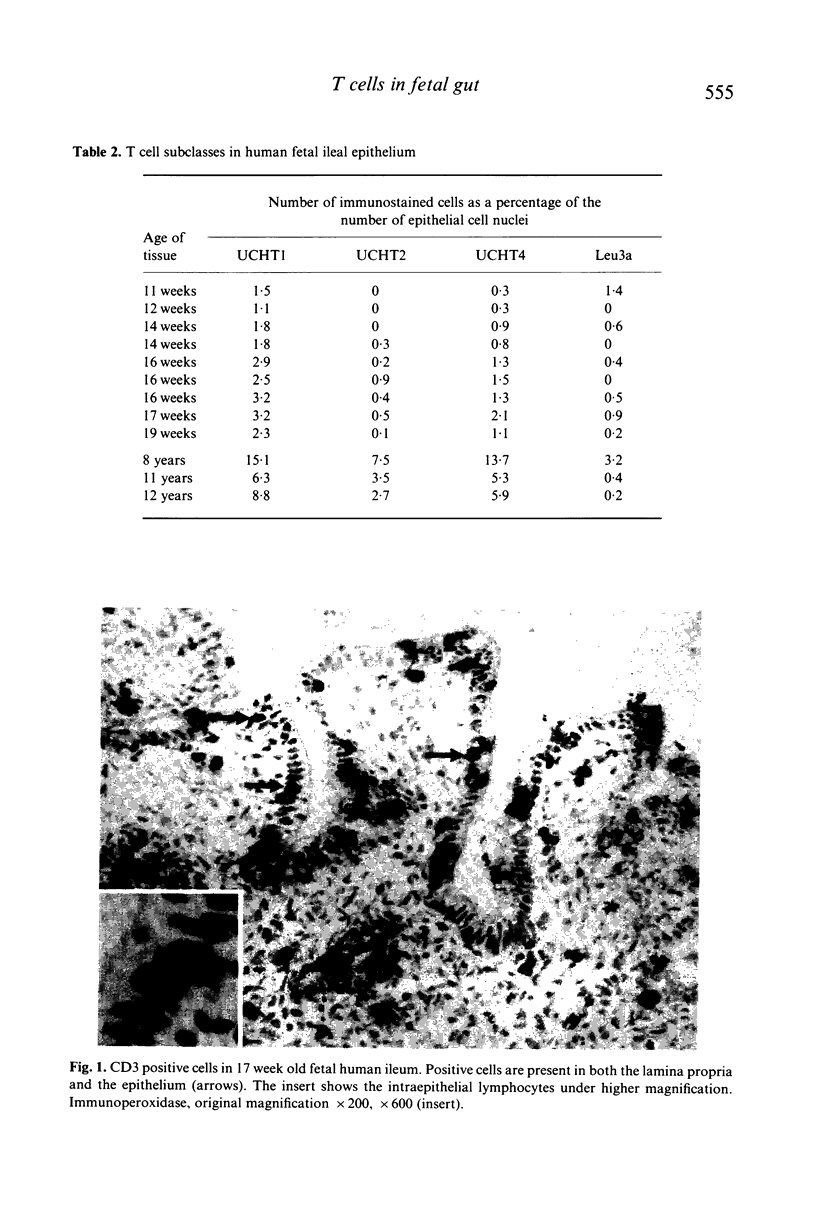
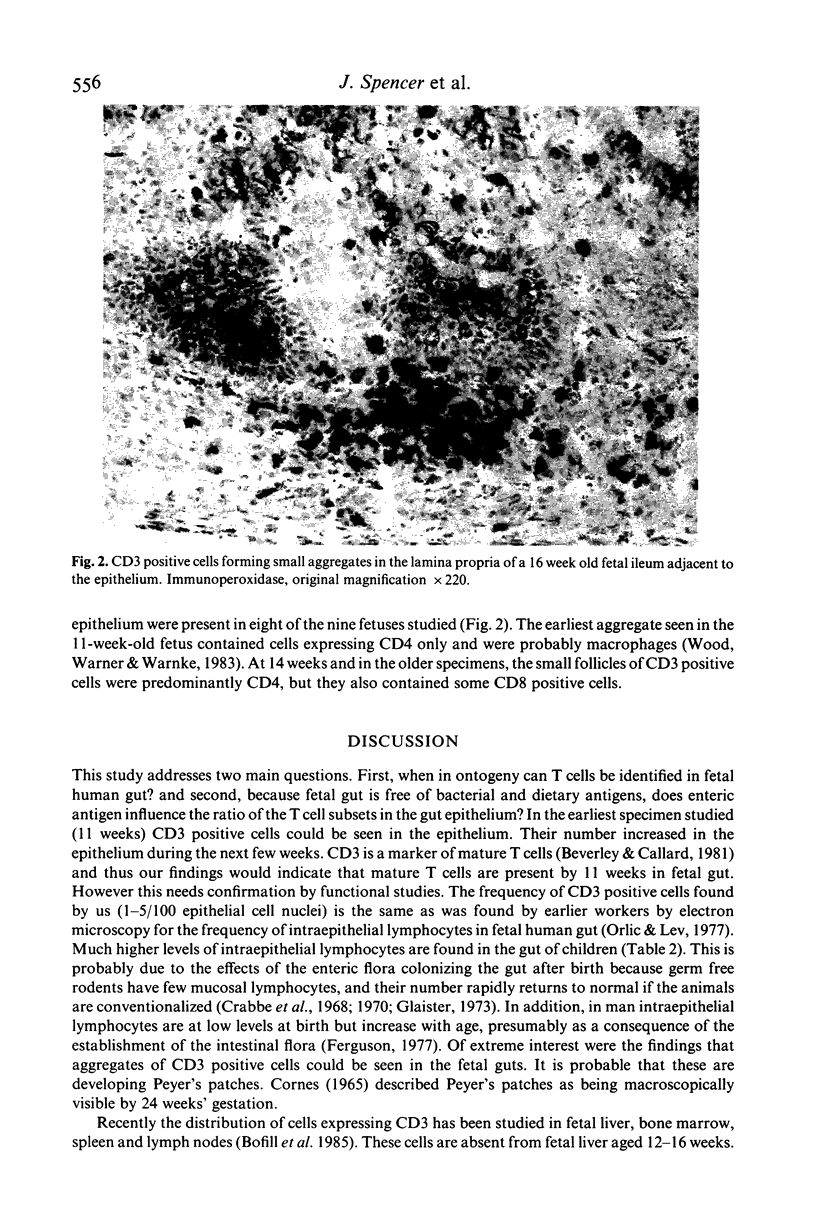
Lymphocytes within fetal human ileum were studied by immunocytochemistry to determine the appearance of T cells in human small intestine and the role of enteric antigen in the accumulation of cells of the suppressor/cytotoxic phenotype in the gut epithelium. In fetal human gut epithelium, cells bearing the pan T cell marker UCHT1 (CD3) were present in all of the specimens studied (11-19 weeks gestation). Of these, UCHT4+ (CD8, suppressor/cytotoxic phenotype) predominated over the leu3a+ (CD4, helper/inducer phenotype), although the differences were not as marked as in postnatal gut. UCHT1+ cells were also present in the lamina propria, frequently as small aggregates beneath the epithelium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Bofill M., Janossy G., Janossa M., Burford G. D., Seymour G. J., Wernet P., Kelemen E. Human B cell development. II. Subpopulations in the human fetus. J Immunol. 1985 Mar;134(3):1531–1538. [PubMed] [Google Scholar]
- Carroll A. M., Reisner Y., de Sousa M. Lyt phenotype and lectin binding properties of mouse lymphocytes which enter lymph nodes. Adv Exp Med Biol. 1982;149:161–165. doi: 10.1007/978-1-4684-9066-4_22. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Quaroni A., Kurnick J. T., Bhan A. K. Intraepithelial lymphocytes modulate Ia expression by intestinal epithelial cells. J Immunol. 1984 May;132(5):2244–2252. [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- Cornes J. S. Number, size, and distribution of Peyer's patches in the human small intestine: Part I The development of Peyer's patches. Gut. 1965 Jun;6(3):225–229. doi: 10.1136/gut.6.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbé P. A., Bazin H., Eyssen H., Heremans J. F. The normal microbial flora as a major stimulus for proliferation of plasma cells synthesizing IgA in the gut. The germ-free intestinal tract. Int Arch Allergy Appl Immunol. 1968;34(4):362–375. doi: 10.1159/000230130. [DOI] [PubMed] [Google Scholar]
- Crabbé P. A., Nash D. R., Bazin H., Eyssen H., Heremans J. F. Immunohistochemical observations on lymphoid tissues from conventional and germ-free mice. Lab Invest. 1970 May;22(5):448–457. [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon S. B., MacDonald T. T. Functional properties of lymphocytes isolated from murine small intestinal epithelium. Immunology. 1984 Jul;52(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Ferguson A. Intraepithelial lymphocytes of the small intestine. Gut. 1977 Nov;18(11):921–937. doi: 10.1136/gut.18.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaister J. R. Factors affecting the lymphoid cells in the small intestinal epithelium of the mouse. Int Arch Allergy Appl Immunol. 1973;45(5):719–730. doi: 10.1159/000231071. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The mouse gut T lymphocyte, a novel type of T cell. Nature, origin, and traffic in mice in normal and graft-versus-host conditions. J Exp Med. 1978 Dec 1;148(6):1661–1677. doi: 10.1084/jem.148.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyscom N., Brueton M. J. Intraepithelial, lamina propria and Peyer's patch lymphocytes of the rat small intestine: isolation and characterization in terms of immunoglobulin markers and receptors for monoclonal antibodies. Immunology. 1982 Apr;45(4):775–783. [PMC free article] [PubMed] [Google Scholar]
- Orlic D., Lev R. An electron microscopic study of intraepithelial lymphocytes in human fetal small intestine. Lab Invest. 1977 Dec;37(6):554–561. [PubMed] [Google Scholar]
- Parrott D. M., Tait C., MacKenzie S., Mowat A. M., Davies M. D., Micklem H. S. Analysis of the effector functions of different populations of mucosal lymphocytes. Ann N Y Acad Sci. 1983 Jun 30;409:307–320. doi: 10.1111/j.1749-6632.1983.tb26879.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal P., Rimm I. J., Umiel T., Griffin J. D., Osathanondh R., Schlossman S. F., Nadler L. M. Ontogeny of human hematopoietic cells: analysis utilizing monoclonal antibodies. J Immunol. 1983 Jul;131(1):232–237. [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Intestinal lymphocyte subpopulations in inflammatory bowel disease: an analysis by immunohistological and cell isolation techniques. Gut. 1984 Jan;25(1):32–40. doi: 10.1136/gut.25.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Lymphocyte subpopulations in the human small intestine. The findings in normal mucosa and in the mucosa of patients with adult coeliac disease. Clin Exp Immunol. 1983 Apr;52(1):219–228. [PMC free article] [PubMed] [Google Scholar]
- Spencer J., Finn T., Isaacson P. G. Gut associated lymphoid tissue: a morphological and immunocytochemical study of the human appendix. Gut. 1985 Jul;26(7):672–679. doi: 10.1136/gut.26.7.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waksman B. H. The homing pattern of thymus-derived lymphocytes in calf and neonatal mouse Peyer's patches. J Immunol. 1973 Sep;111(3):878–884. [PubMed] [Google Scholar]
- Wood G. S., Warner N. L., Warnke R. A. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983 Jul;131(1):212–216. [PubMed] [Google Scholar]