Abstract
Ion compartmentalization is essential for plant growth and development. The Arabidopsis open reading frames for CAX1, CAX2, and CAX3 (cation exchangers 1, 2, and 3) were previously identified as transporters that may modulate ion fluxes across the vacuolar membrane. To understand the diversity and role of H+/cation transporters in controlling plant ion levels, another homolog of the CAX genes, CAX4, was cloned from an Arabidopsis cDNA library. CAX4 is 53% identical to CAX1 at the amino acid level, 42% identical to CAX2, and 54% identical to CAX3. CAX4 transcripts appeared to be expressed at low levels in all tissues and levels of CAX4 RNA increased after Mn2+, Na+, and Ni2+ treatment. An N-terminal CAX4-hemagglutinin fusion appeared to localize to both yeast and plant vacuolar membranes. When expressed in yeast, CAX4, like CAX3, failed to suppress the Ca2+ sensitivity of yeast strains deficient in vacuolar Ca2+ transport. Several modifications to CAX4 allowed the protein to transport Ca2+. Addition of amino acids to the N terminus of CAX4 and CAX3 caused both transporters to suppress the sensitivity of yeast strains deficient in vacuolar Ca2+ transport. These findings suggest that CAX transporters may modulate their ion transport properties through alterations at the N terminus.
Plants have multiple mechanisms to maintain appropriate intracellular levels of various ions (Fox and Guerinot, 1998). Uptake, transport of ions across the plasma membrane, and intracellular sequestration, all regulate the levels of nutrients and toxins in the cytosol. The plant vacuole plays a major role in the intracellular sequestration of various compounds (Marschner, 1995; Marty, 1999). Vacuolar transporters may provide an important mechanism for ion sequestration (Salt and Wagner, 1993; Shaul et al., 1999). In fact, a concentration gradient of Na+, Ca2+, Cd2+, and Mn2+ is established across the vacuolar membrane (tonoplast) by H+/Na+, H+/Ca2+, H+/Cd2+, and H+/Mn2+ exchange activities (Schumaker and Sze, 1985; Salt and Wagner, 1993; Apse et al., 1999; Gonzalez et al., 1999); however, the precise number of genes encoding these biochemical activities and the capacity of the individual transporters to transport various ions remain largely unknown.
Several plant vacuolar cation transporters have been isolated (Hirschi et al., 1996; Shaul et al., 1999). The Arabidopsis transporters, CAX1, CAX2, and CAX3 (cation exchangers 1, 2, and 3), may play a central role in Ca2+ and metal sequestration into the vacuole. CAX1 and CAX2 were identified (Hirschi et al., 1996) by their ability to sequester Ca2+ into yeast vacuoles in Saccharomyces cerevisiae mutants deleted for the vacuolar high-affinity Ca2+-ATPase (PMC1) and low-affinity H+/Ca2+ antiporter (VCX1). Biochemical activities of CAX1 in yeast vacuoles correlate well with those described for the vacuolar H+/Ca2+ antiport activities from plant sources (Hirschi et al., 1996; Ueoka-Nakanishi et al., 1999). CAX2 appears to be a plant vacuolar metal transporter (Hirschi et al., 2000). CAX3 cannot suppress the Ca2+ sensitive phenotype of yeast mutants defective in vacuolar Ca2+ transport and the precise function of this transporter remains unknown (Shigaki and Hirschi, 2000). The Arabidopsis genome also contains several other CAX-like open reading frames (Maser et al., 2001) that may contribute to ion homeostasis.
Using yeast as an experimental tool, two domains have been identified that modulate CAX1 activity (Pittman and Hirschi, 2001; Shigaki et al., 2001). The first domain has been termed the Ca2+ domain, located between amino acids 87 to 95 in CAX1 (Shigaki et al., 2001). CAX3 can suppress the Ca2+ sensitivity of yeast vacuolar Ca2+ transport mutants if this nine-amino acid region of CAX1 is inserted into CAX3 (CAX3–9). Exchanging this nine-amino acid region of CAX1 into CAX2 greatly increased yeast vacuolar Ca2+ transport (CAX2–9). The second domain that regulates CAX function has been termed the regulatory or autoinhibitory domain (Pittman and Hirschi, 2001). The CAX1 open reading frame contains an additional 36 amino acids at the N terminus that were not found in the original “shorter” clone (sCAX1) identified by suppression of yeast vacuolar Ca2+ transport mutants. This “longer” version of CAX1 does not suppress the yeast Ca2+ transport defects, despite localization to the yeast vacuole. Minor alterations in the 36-amino acid region restore H+/Ca2+ transport (Pittman and Hirschi, 2001). Sequence analysis suggests that a 36-amino acid N-terminal regulatory domain may be present in all Arabidopsis CAX-like transporters. These findings suggest structural features involved in regulation of H+/cation antiport; however, the extent to which these regulatory motifs modulate other CAX-like genes has not been addressed.
To investigate the diversity of CAX gene function and the ubiquity of CAX gene regulation, we have cloned an additional CAX homolog, termed CAX4. We monitored the expression of CAX4 in Arabidopsis and monitored localization in yeast and plants using an N-terminal hemagglutinin (HA) tag. We then modified the N terminus and Ca2+ domain of CAX4 in an attempt to modify transport activity in yeast. We made similar modifications to CAX3 and CAX1 and assayed their functions in yeast. These findings offer insights into the modulation of H+/cation antiport in plants.
RESULTS
Identification of CAX4
To further understand the diversity of H+/cation antiporters, we were interested in cloning and characterizing other CAX-like genes from Arabidopsis. The sequence of the Arabidopsis genome suggests that several other putative H+/cation antiporters exist that may function in ion homeostasis (Maser et al., 2001). The open reading frame most similar to the previously characterized CAX genes is located on chromosome 5 and has been designated CAX4. Specific primers were synthesized according to genomic sequence from an Arabidopsis BAC clone F7A7_10 and template DNA was derived from an Arabidopsis Landsberg erecta cDNA library (Minet et al., 1992) to clone CAX4 by a PCR-based approach. The cloned CAX4 open reading frame contained 1,341 nucleotides, which could encode 446 amino acids and produce a putative 49-kD protein (Fig. 1A). The CAX4 open reading frame has many of the signature elements of H+/Ca2+ antiporters characterized from bacteria, fungi and plants (Mäser et al., 2001). CAX4 has approximately the same Mr as many microbial H+/Ca2+ antiporters, and computer-assisted hydropathy analyses generated profiles for CAX4 that are similar to CAX1, CAX2, and CAX3 (Fig. 1B). Like these open reading frames, CAX4 contains a central hydrophilic motif rich in acidic amino acid residues (the acidic motif) that bisects the polypeptide into two groups of approximately equal length. CAX4 is 53% identical (67% similar) to CAX1, 54% identical to CAX3 (69% similar), and 42% identical to CAX2 (52% similar). CAX4 contains an N-terminal hydrophilic region that is similar to the CAX1 autoinhibitory domain (Pittman and Hirschi, 2001). The putative Ca2+ domain of CAX4 does not resemble the Ca2+ domains of the previously characterized CAX genes (Shigaki et al., 2001).
Figure 1.
A, Alignment of deduced amino acid sequences of polypeptides encoded by Arabidopsis CAX 1–4 genes and mung bean VCAX1. Alignments were performed using the ClustalW 1.8 program (Baylor College of Medicine; Thompson et al., 1994). Consensus amino acid residues are boxed in black (identical) or gray (similar). Gaps introduced to maximize the alignments are denoted by hyphens. The 11 putative transmembrane spans (M1–11) predicted for CAX and the central hydrophilic motif rich in acidic residues are overlined. B, Hydropathy profile of Arabidopsis CAXs are predicted over a running window of 15 amino acid residues according to Kyte and Doolittle (1982). Putative transmembrane spans are numbered. The N-terminal regulatory region (NRR), Ca2+ domain (CaD), and acidic motif are indicated. The accession numbers for CAX 1 to -4 and VCAX1 are AF461691, AF424628, AF256229, AF409107, and AB012932, respectively.
Expression of CAX4 in Arabidopsis
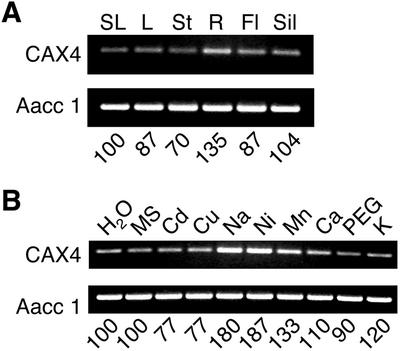
The expression of CAX1 and CAX2 correlates with the transport properties of each transporter in yeast (Hirschi, 1999; Hirschi et al., 2000). That is, the high-affinity, high-capacity H+/Ca2+ transporter CAX1 is highly expressed in response to exogenous Ca2+ in plants, whereas the low-affinity H+/Ca2+ transporter CAX2 is not induced by exogenous Ca2+ in plants. To test the expression of CAX4, standard northern analysis using total RNA was done using the entire CAX4 coding sequence. Using a variety of experimental conditions, no CAX4 transcripts could be detected (data not shown); however, using a more sensitive reverse transcription (RT)-PCR approach, we were able to amplify CAX4 specific cDNA in all tissues analyzed (Fig. 2A). This semiquantitative approach suggests that CAX4 RNA levels increased slightly in response to Mn2+, Ni2+, and Na+, and were not induced by other ions such as Ca2+ (Fig. 2B).
Figure 2.
Expression of CAX4 in Arabidopsis. A, CAX4 transcripts were detected in total RNA from different tissues by RT-PCR. SL, Stem leaf; L, rosette leaf; St, stem; R, root; Fl, flower; Sil, silique. B, CAX4 transcripts were examined by RT-PCR in total RNA from seedlings treated for 12 h with the following solutions: water (as a control), Murashige and Skoog (nutrient media), 0.5 mm CdCl2, 0.1 mm CuSO4, 80 mm NaCl, 0.1 mm NiSO4, 2 mm MnCl2, 100 mm CaCl2, 10% (w/v) polyethylene glycol 3350 solution (118 mOsm/kg), and 80 mm KCl, respectively. Top panel in A and B, a 414-bp CAX4-specific fragment was amplified by RT-PCR. Bottom panel in A and B, a 457-bp actin aac1 gene fragment was amplified by RT-PCR as an internal control. The numbers indicate the relative intensities of the PCR bands that are given in percentages as compared with control (SL as a control in A, water-treated as a control in B).
Expression of CAX4 in Yeast
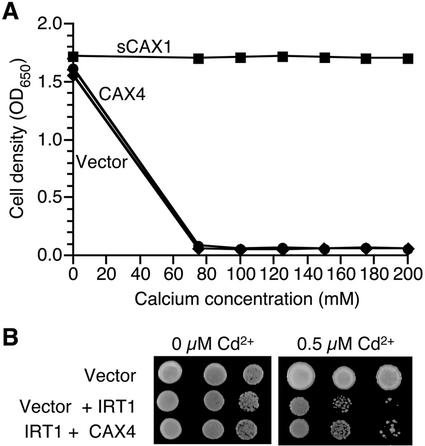
To test the properties of CAX4, we expressed the open reading frame in various yeast strains. The CAX4 cDNA was placed initially under the transcriptional control of the yeast promoter glyceraldehyde phosphate dehydrogenase (GPD) on a high-copy-number yeast plasmid (Nathan et al., 1999). This plasmid was then introduced into yeast strains that contained defects in vacuolar Ca2+ transport to determine whether CAX4 could suppress yeast vacuolar Ca2+ transport mutants in a manner similar to the N-terminal truncated CAX1 (sCAX1) and CAX2 (Hirschi et al., 1996). As shown in Figure 3A, sCAX1 suppresses this growth defect. Under these assay conditions, CAX2 suppresses the Ca2+ sensitivity in a manner indistinguishable from sCAX1 (Hirschi et al., 1996; data not shown); however, like CAX3 (Shigaki and Hirschi, 2000), CAX4 was not able to suppress the Ca2+ sensitivity of yeast strains deficient in vacuolar Ca2+ transport.
Figure 3.
A, Ca2+ sensitivity assay of yeast K667 strains expressing vector, sCAX1 and CAX4, respectively. Cells were diluted 10-fold into fresh media containing a range of CaCl2 concentrations and incubated for 1 d at 30°C in flat-bottom 24-well dishes (1.0 mL/well). Optical density at 650 nm was measured for each resuspended culture. B, Cd2+ sensitivity assay of IRT1 expressing yeast strains. The yeast strains, DY1457/FL61(wt) and DY1457/IRT1, were transformed with vector and CAX4. The indicated yeast strains were grown in liquid media and then a series of dilutions were spotted on to the control and Cd2+-containing media.
We also tested the possibility that CAX4 transports other metals, thus making the host yeast strains tolerant (or hypersensitive to) to these metals. Expression of CAX4 in a Mg2+ requiring strain (CM66) did not suppress the ion sensitivity of this strain (MacDiarmid and Gardner, 1998). A range of metals, such as Al3+, Cd2+, Cu2+, Ni2+, Na+, and Zn2+, was tested with a K661 yeast strain expressing CAX4. For such metal sensitivity assays, this yeast strain is indistinguishable from wild-type strains in our assay conditions (Shigaki and Hirschi, 2000). CAX4 expression did not alter the yeast growth on any media except Cd2+ (data not shown). An Arabidopsis plasma membrane ion transporter, IRT1, can transport Cd2+ into the cytosol, and when expressed in wild-type yeast strains (DY1457), it causes the strain to be more sensitive to Cd2+ (Rogers et al., 2000; Fig. 3B). When CAX4 was expressed in the yeast strain harboring IRT1, CAX4 was able to partially rescue the Cd2+ sensitivity of the IRT1-expressing strain (Fig. 3B).
Localization of CAX4 in Yeast
The inability of CAX4 to suppress the Ca2+ sensitivity of yeast mutants defective in vacuolar Ca2+ transport could be due to targeting of the protein to a different membrane. To identify the cellular location of CAX4 in yeast, an epitope-tagged variant was generated by the fusion of a triple copy of HA to the N terminus (HA:CAX4). An N-terminal HA epitope tag has previously been used to identify the cellular location of VCX1, CAX1, and CAX3 in yeast (Cunningham and Fink, 1996; Pittman and Hirschi, 2001; Shigaki et al., 2001). As shown in Figure 4, western-blot analysis of yeast membranes fractionated on Suc gradients showed that CAX4 cofractionated with vacuolar membranes. The distribution of HA:CAX4 corresponded with that of HA:sCAX1 and the yeast vacuolar membrane marker, alkaline phosphatase.
Figure 4.
Subcellular localization of CAX4 in yeast. Saccharomyces cerevisiae K667 strains were transformed with plasmid DNA containing HA:sCAX1 and the HA:CAX4 fusion. Microsomal membranes were extracted from HA:sCAX1- and HA:CAX4-expressing yeast cells and fractionated in a linear Suc gradient. Ten micrograms of total proteins from the numbered fractions were separated on 12% (w/v) SDS-PAGE and transferred onto nitrocellulose membrane. Western blotting analyses were performed using the following primary antibodies: monoclonal mouse IgG against HA epitope and monoclonal mouse IgG against yeast vacuolar alkaline phosphatase (V-ALP), which is used as an indicator of yeast vacuolar membranes. The numbers indicate the Suc concentration of each fraction.
Localization of CAX4 in Transgenic Tobacco
The localization of HA:CAX4 on the vacuolar membrane of plants was shown by the heterologous expression of the CAX4 fusion protein in a suspension of tobacco cells. The fusion protein under the control of the cauliflower mosaic virus 35S promoter was expressed in tobacco BY-2 cells. In the case of BY-2 cells, many vacuoles are generally observed in a single cell (Kost et al., 1998). As shown in Figure 5, A and B, confocal images of red fluorescent signals stained by Texas Red conjugated antibody revealed that HA:CAX4 is localized in the vacuolar membrane.
Figure 5.
Expression of HA:CAX4 in tobacco BY-2 cells. A and B, Immunostaining of HA:CAX4 in BY-2 cell. A, HA:CAX4 was detected on vacuolar membrane by HA antibody and Texas Red conjugated secondary antibody (indicated by arrow). B, A superimposed image of red channel and transmitted light demonstrated only vacuolar membranes were stained (indicated by arrow). Bars = 50 μm. C, Immunoblotting of HA:CAX4 in tobacco BY-2 cells. Western blot was performed as described in “Materials and Methods.” Monoclonal antibodies against HA and a plant endoplasmic reticulum luminal protein (BiP), an indicator of endoplasmic reticulum, were used at dilutions of 1:1,000 and 1:1,500, respectively. Polyclonal antibodies against mung bean (Vigna radiata) vacuolar pyrophosphatase (V-PPase) and radish plasma membrane aquaporin (PAQ1) were used at 1:1,000 dilutions. The numbers indicate the concentration of the Suc fraction.
To confirm vacuolar membrane localization of the fusion protein, the crude membrane fraction of tobacco cells was subjected to Suc gradient centrifugation and immunoblotting with the anti-HA antibody. The distribution of the fusion protein (fractions from 26% to 33%) was paralleled by that of a vacuolar marker (mung bean vacuolar pyrophosphatase) and not that of an endoplasmic reticulum marker (plant endoplasmic reticulum luminal protein) and a plant plasma membrane marker (radish plasma membrane aquaporin; Fig. 5C).
Alterations in CAX4 That Facilitate Yeast Vacuolar Ca2+ Transport
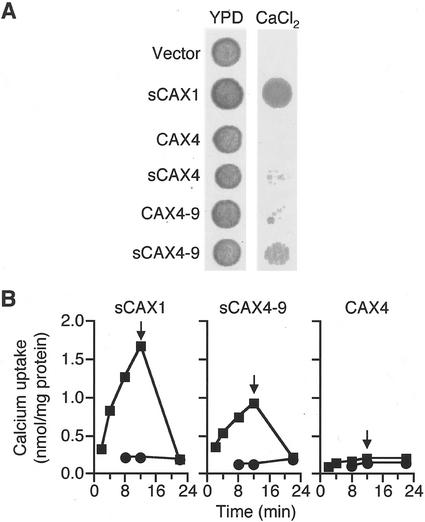
Given that the N terminus appears to act as an autoinhibitory domain for CAX1 Ca2+ transport in yeast, we were interested in testing whether the N terminus of CAX4 acts as an autoinhibitory domain. Truncated and, thus, possibly active, versions of CAX4 (sCAX4) should not be present in cDNA libraries because the CAX4 open reading frame, unlike the CAX1 and CAX2 open reading frames, does not contain a Met residue after the putative autoinhibitory domain. We, thus, created a truncated version of CAX4 by deleting the first 37 amino acids of CAX4 and replacing the Ser residue at position 38 with a Met residue. Yeast strains expressing sCAX1 grow well in the Ca2+-containing media, whereas the sCAX4 expressing cells grow poorly (Fig. 6A). The growth of several sCAX4 expressing cells is meaningful when compared with the complete absence of growth for CAX4 or vector expressing yeast cells (Fig. 6A).
Figure 6.
Suppression of Ca2+ hypersensitivity and Ca2+ uptake assay. A, Yeast assay for suppression of Ca2+ hypersensitivity by various CAX4 proteins. Yeast K667 strain was transformed with plasmid DNA containing vector, sCAX1, CAX4, sCAX4, CAX4–9, and sCAX4–9. Yeast strains were grown to OD650 of 1.0 in selection media at 30°C. Cells were diluted 5-fold and 5 μL of each dilution was spotted on yeast peptone dextrose (YPD) plate supplemented with 175 mm CaCl2 and incubated for 2 d at 30°C. B, H+/Ca2+ transport of sealed enriched vesicles purified from yeast expressing CAX4, sCAX1, and sCAX4–9. Ca2+ uptake was examined with 10 μm total CaCl2 containing 45Ca in the presence (●) and absence (▪) of 5 μm gramicidin. The Ca2+ ionophore A23187 was added at 12 min to a concentration of 5 μm (indicated by arrow).
Insertion of the CAX1 Ca2+ domain into CAX3 allows yeast cells expressing this chimeric construct to suppress vacuolar Ca2+ transport defects (Shigaki et al., 2001). Given that this region of CAX4 is unlike CAX1, CAX2, or CAX3, we proceeded to change the 11 amino acids in this region to the amino acids found in the CAX1 Ca2+ domain. Although we have altered 11 amino acids, we have termed this construct CAX4–9 so that the nomenclature will be consistent with similar modifactions made in CAX2 and CAX3 (Shigaki et al., 2001). Yeast cells expressing CAX4–9 grew weakly in Ca2+ containing media at a rate comparable with sCAX4 expressing cells (Fig. 6A).
We then created chimeric CAX4 constructs containing both the N-terminal truncation and the CAX1 Ca2+ domain (sCAX4–9). Yeast cells expressing sCAX4–9 suppressed the vacuolar Ca2+ transport defects in a manner indistinguishable from CAX1 variants, which lack the N-terminal autoinhibitory domain (Fig. 6A).
To confirm that the Ca2+ tolerance observed for the sCAX4–9 expressing yeast strain was due to a restoration of Ca2+ transport activity, H+/45Ca2+ transport was measured from endomembrane vesicles obtained from sCAX4–9-expressing yeast cells. H+/Ca2+ transport by sCAX4–9 was measured using 10 μm 45CaCl2, and was comparable with the activity observed for sCAX1-containing vesicles, whereas transport was not found in vesicles harboring CAX4 (Fig. 6B).
N-Terminal Additions Facilitate CAX-Mediated Ca2+ Transport
Alterations in the N terminus can alter the function of many proteins, including the soluble insulin-like growth factor-I. Even additional N-terminal residues can alter the activity of this growth regulator (Tomas et al., 1997). We were interested in determining whether N-terminal additions could also alter the activity of CAX4 or the other CAX transporters. Due to the localization studies, we already had a series of HA-tags fused to the N terminus of the CAX genes. As shown in Figure 7, addition of HA allows CAX4-expressing yeast cells to suppress vacuolar Ca2+ transport mutations in a manner indistinguishable from sCAX1 expression.
Figure 7.
Yeast assay for suppression of Ca2+ hypersensitivity by CAX proteins with N-terminal additions. K667 yeast strain was transformed with vector, sCAX1, CAX1, HA:CAX1, nub:CAX1, CAX3, HA:CAX3, CAX4, HA:CAX4, and rCAX4. A single colony of each transformant was streaked on YPD plate supplemented with 175 mm CaCl2 and incubated at 30°C for 2 d.
To verify that HA:CAX4 suppression was due to the altered conformation of CAX4 and not expression of the HA, we made a CAX4 construct containing 10 arbitrary amino acids fused to the N terminus (rCAX4). Yeast cells expressing rCAX4 weakly suppressed the vacuolar Ca2+ transport mutations. This growth was subtle and like sCAX4 and CAX4–9 could be monitored by the growth of small individual yeast colonies on Ca2+ containing media (Fig. 7). This growth on Ca2+ containing media was significantly weaker than the HA:CAX4 growth but was meaningful compared with the complete lack of yeast growth by vector expressing cells.
Expression of HA:CAX1 and HA:CAX3 in yeast also suppressed the vacuolar Ca2+ transport defects. Furthermore, expression of the N-terminal one-half of ubiquitin fused to CAX1 at the N terminus (nub:CAX1) also suppressed the yeast mutations. Suppression with HA:CAX1 or nub:CAX1 was consistently stronger than HA:CAX3 suppression (Fig. 7). H+/Ca2+ transport activity was observed from HA:CAX1, HA:CAX3, HA:CAX4, and nub:CAX1 vesicle preparations (data not shown).
DISCUSSION
H+/cation antiporter activity has been described in numerous plant species and on several membranes, including the vacuolar, plasma, and chloroplast thylakoid membranes (Schumaker and Sze, 1985; Blumwald and Poole, 1986; Kasai and Muto, 1990; Ettinger et al., 1999; Shaul et al., 1999). This study was carried out to investigate the diversity of the genes that may confer these activities in plants. This study reports the identification and partial characterization of an Arabidopsis CAX gene, CAX4, which has greater than 50% identity to the previously characterized H+/Ca2+ transporter CAX1. CAX4 has extensive sequence homology with all the previously characterized H+/Ca2+ antiporters (Ueoka-Nakanishi et al., 1999, 2000; Hirschi, 2001). The expression pattern of CAX4 in plants suggests that this gene is expressed at levels lower than CAX1, CAX2, or CAX3. Like the metal transporter CAX2, CAX4 RNA levels were not induced by exogenous Ca2+ (Hirschi et al., 2000). Like CAX3 and CAX1, CAX4 levels increase with some ion imbalances (Fig. 2; Mn2+, Na+, and Ni2+ treatment). Although the induction of CAX4 after these stimuli is less than 2-fold, this infers that CAX4 may be capable of adjusting specific cytosolic ion concentrations.
CAX4 appeared to be highly expressed in yeast and N-terminal HA-tagged versions of CAX4 localized at the yeast vacuole (Fig. 4). Furthermore, the plant vacuolar membrane location of CAX4 was shown by N-terminal HA-tagged CAX4 in tobacco cells (Fig. 5). This is a useful system for morphologically identifying the localization of proteins, although it should be noted that, in this experiment, the HA:CAX4 fusion protein was expressed under artificial conditions. The HA-tag was observed predominantly in the vacuolar membrane and not in the plasma membrane or nuclear envelope. This distribution was confirmed in immunoblot experiments and is consistent with the expression of sGFP-VCAX1 in mung bean (Ueoka-Nakanishi et al., 2000).
Conceivably, the N terminus could target the CAX proteins to various membranes in yeast and plants, and the HA-tag or other modifications could perturb intracellular targeting and cause the transporters to localize by default to the vacuolar membrane. Several observations suggest that this is not the case. (a) Expression of chimeric constructs containing the N terminus of CAX3 fused to CAX1 suppress yeast mutants defective in vacuolar Ca2+ transport (J.K. Pittman and K.D. Hirschi, unpublished data). This strongly implies that the CAX3 N terminus is not localizing proteins to a membrane other than the vacuolar membrane. (b) Removal of the N terminus of CAX3 or CAX4 does not allow strong suppression of the vacuolar Ca2+ transport defects. This suggests that the N terminus is not directing CAX3 or CAX4 to a membrane other than the vacuole for Ca2+ transport. (c) Expression of the full-length vacuolar H+/Ca2+ transporter from mung bean, VCAX1, suppresses yeast mutants defective in vacuolar Ca2+ transport. Given that the N terminus of VCAX1 is similar to CAX1, CAX2, CAX3, and CAX4, it is unlikely that this sequence targets the mung bean transporter to the vacuolar membrane and the Arabidopsis proteins are being targeted to a different membrane.
The CAX4 gene product does not suppress yeast defects in vacuolar Ca2+ transport. However, CAX4 expression weakly suppressed the Cd2+ sensitivity of an IRT1 harboring strain. Although this suppression was not strong, it suggests that CAX4 functions in providing Cd2+ tolerance in this strain, indicating that CAX4 may transport Cd2+. The lack of Ca2+ activity of CAX4 in yeast resembles CAX3 (Shigaki and Hirschi, 2000). Expression of CAX4 N-terminal truncations or CAX4 chimeric constructs containing the CAX1 Ca2+ domain both very weakly suppress the yeast vacuolar Ca2+ transport defects. This suppression consists of only a few isolated colonies growing on the Ca2+ containing media (Fig. 6A). When these two alterations are combined, expression of the chimeric CAX4 constructs strongly suppressed the yeast mutations. From these observations, it appears that removal of the N terminus or alterations of the Ca2+ domain alone are insufficient for high levels of CAX4 mediated Ca2+ transport. Juxtaposed with these observations was the finding that N-terminal amino acid additions alone were sufficient to mediate CAX4, CAX3, or CAX1 H+/Ca2+ transport in yeast (Fig. 7). This suggests that repositioning of the N terminus relative to the native Ca2+ domain of CAX3 and CAX4 is sufficient to mediate H+/Ca2+ transport.
Most CAX genes appear to be expressed in all tissues. The biological significance of the fact that truncated forms of CAX1 and CAX2 can suppress yeast mutants defective in vacuolar Ca2+ transport is not immediately clear. A truncated version of CAX3 cannot suppress this phenotype, whereas truncated CAX4 variants weakly suppress the phenotype; however, both CAX3 and CAX4 can suppress this phenotype if N-terminal extensions are present (Fig. 7). Thus, CAX3 and CAX4 contain the necessary components to drive yeast vacuolar Ca2+ transport; however, we must now address the questions of how and when these transporters function as vacuolar Ca2+ transporters in plants.
MATERIALS AND METHODS
All novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material.
Plant Materials and Yeast Strains
Arabidopsis ecotype Columbia was used in this study. Seeds were germinated in artificial soil (Metro-Mix 200, Scotts-Sierra, Maysville, OH) and grown and treated with different metal solutions as previously described (Hirschi, 1999).
Saccharomyces cerevisiae strains K661 MATa vcx1^ and K667 (cnb1::Leu1 pmc1::TRP1 vcx1) were used. Those strains have the following additional mutations: ade2-1, can1-100, his3-11, 15leu2-3, 112trp1-1 and ura3-1 (Cunningham and Fink, 1996). The wild-type yeast strain, W301A, and the magnesium-requiring strain, CM66, were used for metal sensitivity assay (Wallis et al., 1989; MacDiarmid and Gardner, 1998). The yeast strain CM66 (alr1::HIS3 alr2::TRP1 his3-200 ura3-52 leu2-1 lys2-202 trp1-63) was obtained from Dr. Richard Gardner (University of Auckland). The pFL61/IRT1 in DY1457 and pFL61 alone in DY1457 yeast strains were obtained from Dr. Erin Connolly (University of South Carolina, Columbia). Yeast cells were transformed using the lithium acetate method and selected on synthetic complete minus His (SC-His) media (Sherman et al., 1986). For Ca2+ tolerance assays, yeast were grown at 30°C for 1 to 3 d on solid YPD medium containing 2% (w/v) yeast extract (DIFCO Laboratories, Detroit), 1% (w/v) bacto-peptone, and 2% (w/v) dextrose, and supplemented with 175 or 200 mm CaCl2 (Hirschi et al., 1996). We used 175 mm CaCl2 in some experiments because the HA:CAX4 expressing strains grew poorly at 200 mm CaCl2. The liquid Ca2+ tolerance assays were done as previously described (Pittman and Hirschi, 2001).
Isolation of CAX4
CAX4 was identified on a BAC clone F7A7_10 (GenBank accession no. AL161946). To clone the full-length cDNA of this gene, two oligonucleotide primers that are complementary to the 5′ and 3′ ends of the predicted CAX4 gene were generated: CAX4 forward primer, 5′-TCTAGAAGATGTCTTCAATCAGTACGG-3′ and CAX4 reverse primer: 5′-GGCGAGCTCTTATCAAAAGAGAAGCTTACTTGA-3′. XbaI and SacI sites (underlined) were introduced for subcloning. Two micrograms of an Arabidopsis Landsberg erecta cDNA library (Minet et al., 1992) was screened using the CAX4 specific primers. The conditions for amplification were 95°C for 2 min followed by 35 cycles at 94°C for 30 s, 60°C for 30 s, 72°C for 2 min, and 72°C for 10 min. The amplified fragments were gel-purified using QIAGENE MAX (Qiagen USA, Valencia, CA) and cloned into pGEM-T-easy vector (Promega, Madison, WI). Multiple clones were completely sequenced. CAX4 cDNAs were also amplified from cDNA pools that were extracted from Arabidopsis seedling (Col. Ecotype; see below). All clones identified were identical to the original clone isolated from the cDNA library.
RNA Extraction and RT-PCR
RNA was isolated using the RNeasy Plant Kit (Qiagen USA), according to the manufacturer's instructions. RNA samples were treated with DNase to minimize any contamination of genomic DNA. Quantitative RT-PCR was performed to detect CAX4 mRNA transcript. The first strand of cDNA was synthesized using 0.2 μg of total RNA as template in 20 μL of reaction mixture, which included 0.5 μg of oligo(dT)(12–18) primer and 200 units of Superscript II transcriptase (Invitrogen, Carlsbad, CA). One microliter of the first strand cDNA was used to amplify a CAX4 gene-specific fragment and an actin 1 fragment (Geisler et al., 2000). Sense and antisense primers for CAX4 and actin 1 were used at concentrations of 0.5 μm each in amplification conditions identical to those used to isolate CAX4 (5′-TCTAGAAGATGTCTTCAATCAGTACGG-3′ and 5′-CCACATGTGGCGTTCATTAAT-3′ [CAX4] and 5′-GTGCTCGACTCTGGAGATGGTGTG-3′ and 5′-CGGCGATTCCAGGGAACATTGTGG-3′ [actin 1]). PCR products were run on 2.5% (w/v) agarose gels and stained with ethidium bromide. The gels were photographed with a digital camera (Kodak, Rochester, NY), and the net intensity of individual PCR products was determined using Kodak ID 2.02 analysis software. The relative intensities in different lanes within each individual experiment were independent of the number of PCR cycles performed.
Construction of Modified CAX Constructs
The truncated CAX4 gene was amplified with sCAX4 forward primer: 5′-GCTCTAGAGGATCCGAGATGGCGTCGTCGTTGATAAGGAAG-3′ and CAX4 reverse primer as described above.
To fuse the triple HA epitope (YPYDVPDYA) to the N terminus of CAX4, HA fragment and CAX4 fragment were generated, respectively, using the following primer sets: HA-5: 5′-GAATTCTCTAGAATGGGCCGCATCTTTATCCCATACGAT-3′ and HA-3: 5′-CCGTACTGATTGAAGACATGCACTGAGCAGCGTAATC-3′ for HA fragment; CAX4–5: 5′-AGATTACGCTGCTCAGTGCATGTCTTCAATCAGTACG-3′ and CAX4 reverse primer as described above for CAX4. HA:CAX4 fusion fragment was amplified with HA-5 and CAX4 reverse primer.
Mutagenesis was performed to replace the 11 amino acid residues of the Ca2+ domain of CAX4 with that of CAX1 (Shigaki and Hirschi, 2001) using primers: 5′-GAATTCCGTCTCCCGCCATTATTTGCACCTATTGTGGCGTCAGTCAGCCTTGGGTCTTTGCGTTGAGC-3′ and 5′-GAATTCCGTCTCCGGCGAGAGGAACGGCCGGGAAG-3′. The type IIS restriction enzyme BsmBI site is underlined. The bold letters indicate the introduced CAX1 11 amino acid residues. The italic letters indicated the CAX4 sequences.
To generate an in-frame fusion of 10 arbitrary amino acid residues to N terminus of CAX4, mutagenesis was performed as described above. The following primer was used to create the 10 arbitrary amino acid residues at the N terminus of CAX4 in piHGpd: 5′-GAA TTC CGT CTC CCG CCG ATG (AGC) NN (GAC) NN (CGA) NN (AGC) NN (GAC) NN (CGA) NN (GCA) NN (CAG) NN (ACG) NN (CGA) NN ATG TCT TCA ATC AGT ACG G-3′. The type IIS restriction enzyme BsmBI site is underlined. The bold letters indicate the introduced 10 arbitrary amino acid residues. The italic letters indicated the CAX4 sequences (Shigaki and Hirschi, 2001).
All PCR amplifications were performed using Expand High Fidelity PCR System (Roche Molecular Biochemicals, Indianapolis), and products were cloned into pGEM-T-easy vector (Promega, Madison, WI). Multiple clones of each construct were sequenced. The gene fragments of CAX4, CAX4 variants and HA:CAX4 were subcloned into the yeast expression vector piHGpd (Nathan et al., 1999) at XbaI/SacI sites for expression in yeast (Shigaki and Hirschi, 2000).
To generate an in-frame fusion of N-terminal ubiquitin (nub) to CAX1, the 37 amino acids of nub were amplified by PCR from plasmid pNubG-ALG5 (Stagljar et al., 1998; obtained from Dr. I. Stagljar, University of Zurich-Irchel) using the forward primer (5′-CGC GGA TCC ATG CAG ATT TTC GTC AAG ACT-3′) and reverse primer (5′-AAT GCC ATG GAG GGA TAC CTT CCT TGT CTT G-3′). A BamHI site (underlined) was generated into the forward primer, and an NcoI site (underlined) was generated into the reverse primer. CAX1 was amplified by PCR from the original CAX1 cDNA clone in pBluescript using the forward primer (5′-CGC GGA TCC AAA ACC ATG GAT GGC GGG AAT CGT GAC AGA GCC GTG G-3′) and reverse primer (5′-AAC GAG CTC TTA TCT AGA TGA GAA AAC TCC TCC TCC TGT TGC A-3′). A BamHI site (underlined) and an NcoI site (double underlined) were generated into the forward primer, and a SacI site (underlined) was generated into the reverse primer. CAX1 was cloned into the BamHI-SacI site of the yeast expression vector piHGpd. To fuse nub to the 5′ end of CAX1, nub was subcloned into the BamHI-NcoI site of CAX1-piHGpd, generating nub:CAX1.
Membrane Fractionation and Western Analysis
Microsomal membranes were prepared from yeast expressing HA:CAX1 and HA:CAX4, essentially as described by Hwang et al. (2000), with a few modifications (Pittman and Hirschi, 2001). Immunoblots were performed and the HA epitope was detected essentially as described previously (Hirschi et al., 1998; Pittman and Hirschi, 2001).
Preparation of Microsomal Membrane-Enriched Vesicles from Yeast Cells
Microsomal membrane-enriched vesicles were isolated from the yeast cells expressing various constructs (Pittman and Hirschi, 2001; Shigaki et al., 2001), essentially as described by Nakanishi et al. (2001).
Ca2+ Uptake Assay
Time-dependent H+/Ca2+ transport into endomembrane vesicles was examined using the direct filtration method (Hwang et al., 1997), with a minor modification as described previously (Salt and Wagner, 1993; Pittman and Hirschi, 2001; Shigaki et al., 2001). Cold CaCl2 (10 μm) including 45Ca2+ (carrier free, American Radiolabeled Chemicals, St. Louis) was used in this examination.
Expression of HA:CAX4 in Tobacco Cells
The coding region of HA:CAX4 was cloned into pBIN19 (CLONTECH, Palo Alto, CA), which contained the cauliflower mosaic virus 35S-promoter fragment and nos terminator (Hull et al., 2000). The recombinant plasmids, or vector controls, were transformed into tobacco (Nicotiana tabacum cv BY-2) cells mediated by Agrobacterium tumefaciens (strain LBA4404; Invitrogen) as previously described (Matsuoka and Nakamura, 1991). To determine the subcellular localization of CAX4 in plant cells, transformed BY-2 cells (HA:CAX4) were subcultured for 4 d and then fixed with 3.7% (w/v) formaldehyde in 50 mm Na2HPO4 overnight at 4°C. The fixed cells were washed three times with 1× phosphate-buffered saline (pH 7.0) and dried on slides, and then treated with 0.5% (w/v) Triton X-100 for 5 min. Cells were blocked with 3% (w/v) bovine serum albumin and then applied with monoclonal antibody against HA at a 1:500 dilution. After treatment with primary antibody, cells were incubated with rabbit IgG against mouse IgG conjugated with Texas Red (Molecular Probes, Eugene, OR), washed four times with 1× phosphate-buffered saline, and then mounted with Aqua Poly/Mount (Polysciences Inc., Warrington, PA). The images were observed and captured by laser scanning biological microscope (Fluoview, Olympus America Inc., Melville, NY; Cheng et al., 2000). To determine the intracellular localization of the fusion protein by immunoblotting, the transformed cells subcultured for 4 d were homogenized and treated as previously described (Hirschi et al., 2000; Ueoka-Nakanishi et al., 2000). Detection of the marker proteins was performed as described (Hong et al., 1999; Shaul et al., 1999; Hirschi et al., 2001; Suga et al., 2001). Enhanced chemiluminescence was performed according to the instructions given by the manufacturer (Amersham, Buckinghamshire, UK). To ensure reproducibility of the results obtained from immunoblots, at least three independent experiments were performed at exposure times which varied from 2 to 30 min.
ACKNOWLEDGMENTS
We thank Maarten J. Chrispeels, Masayoshi Maeshima, Jeffrey Harper, and Orit Shaul for antibodies. We also thank Erin Connolly for the IRT1-expressing DY1457 yeast strain and Richard S. Nelson for tobacco BY-2 suspension cells.
Footnotes
This work was supported by the U.S. Department of Agriculture/Agricultural Research Service (cooperative agreement no. 58–6250–6001), by Phillip Morris USA, and by the National Institutes of Health (grant nos. CHRC 5 P30 and 1R01 GM57427).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010857.
LITERATURE CITED
- Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;285:1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- Blumwald E, Poole RJ. Kinetics of Ca2+/H+ antiport in isolated tonoplast vesicles from storage tissue of Beta vulgaris L. Plant Physiol. 1986;80:727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Su CL, Carter SA, Nelson RS. Vascular invasion routes and systemic accumulation patterns of tobacco mosaic virus in Nicotiana benthamiana. Plant J. 2000;23:349–362. doi: 10.1046/j.1365-313x.2000.00788.x. [DOI] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+-ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger WF, Clear AM, Fanning KJ, Peck ML. Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol. 1999;119:1379–1385. doi: 10.1104/pp.119.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox TC, Guerinot ML. Molecular biology of cation transport in plants. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:669–696. doi: 10.1146/annurev.arplant.49.1.669. [DOI] [PubMed] [Google Scholar]
- Geisler M, Frangne N, Gomes E, Martinoia E, Palmgren MG. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 2000;124:1814–1827. doi: 10.1104/pp.124.4.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Koren'kov V, Wagner GJ. A comparison of Zn, Mn, Cd, and Ca transport mechanisms in oat root tonoplast vesicles. Physiol Plant. 1999;106:203–209. [Google Scholar]
- Hirschi KD. Expression of Arabidopsis CAX1 in tobacco: altered calcium homeostasis and increased stress sensitivity. Plant Cell. 1999;11:2113–2122. doi: 10.1105/tpc.11.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi K. Vacuolar H+/Ca2+ transport: Who's directing the traffic? Trends Plant Sci. 2001;6:100–104. doi: 10.1016/s1360-1385(00)01863-x. [DOI] [PubMed] [Google Scholar]
- Hirschi KD, Korenkov V, Wilganowski N, Wagner G. Expression of Arabidopsis CAX2 in tobacco: altered metal accumulation and increased manganese tolerance. Plant Physiol. 2000;124:125–133. doi: 10.1104/pp.124.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD, Miranda M, Wilganowski N. Phenotypic changes in Arabidopsis caused by expression of a yeast vacuolar H+/Ca2+ antiporter. Plant Mol Biol. 2001;46:57–65. doi: 10.1023/a:1010620227913. [DOI] [PubMed] [Google Scholar]
- Hirschi KD, Zhen R-G, Cunningham KW, Rea PA, Fink GR. CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA. 1996;93:8782–8786. doi: 10.1073/pnas.93.16.8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, D'Amore PA. PDGF, TGF-β, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J Cell Biol. 1998;141:805–814. doi: 10.1083/jcb.141.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong B, Ichida A, Wang Y, Gens JS, Pickard BG, Harper JF. Identification of a calmodulin-regulated Ca2+-ATPase in the endoplasmic reticulum. Plant Physiol. 1999;119:1165–1175. doi: 10.1104/pp.119.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull A, Vij R, Celenza JL. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc Natl Acad Sci USA. 2000;97:2379–2384. doi: 10.1073/pnas.040569997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Harper JF, Liang F, Sze H. Calmodulin activation of an endoplasmic reticulum-located calcium pump involves an interaction with the N-terminal autoinhibitory domain. Plant Physiol. 2000;122:157–168. doi: 10.1104/pp.122.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Ratterman DM, Sze H. Distinction between endoplasmic reticulum-type and plasma membrane-type Ca2+ pumps. Plant Physiol. 1997;113:535–548. doi: 10.1104/pp.113.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai N, Muto S. Ca2+ pump and Ca2+/H+ antiporter in plasma membrane vesicles isolated by aqueous two-phase partitioning from maize leaves. J Membr Biol. 1990;114:133–142. doi: 10.1007/BF01869094. [DOI] [PubMed] [Google Scholar]
- Kost BS, Spielhofer P, Chua NH. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 1998;16:393–401. doi: 10.1046/j.1365-313x.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- MacDiarmid CW, Gardner RC. Overexpression of the Saccharomyces cerevisiae magnesium transport system confers resistance to aluminum ion. J Biol Chem. 1998;273:1727–1732. doi: 10.1074/jbc.273.3.1727. [DOI] [PubMed] [Google Scholar]
- Marschner H. Mineral Nutrition on Higher Plants. San Diego: Academic Press; 1995. [Google Scholar]
- Marty F. Plant vacuoles. Plant Cell. 1999;11:587–599. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D et al. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol. 2001;126:1646–1667. doi: 10.1104/pp.126.4.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Nakamura K. Properties of a precursor to a plant vacuolar protein required for vacuolar targeting. Proc Natl Acad Sci USA. 1991;88:834–838. doi: 10.1073/pnas.88.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet M, Dufour M-E, Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992;2:417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Saijo T, Wada Y, Maeshima M. Mutagenic analysis of functional residues in putative substrate-binding site and acidic domains of vacuolar H+-pyrophosphatase. J Biol Chem. 2001;276:7654–7660. doi: 10.1074/jbc.M009743200. [DOI] [PubMed] [Google Scholar]
- Nathan DF, Vos MH, Lindquist S. Identification of SSF1 and HCH1 as multicopy suppressors of a Saccharomyces cerevisiae Hsp90 loss-of-function mutation. Proc Natl Acad Sci USA. 1999;96:1409–1414. doi: 10.1073/pnas.96.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman JK, Hirschi KD. Regulation of CAX1, an Arabidopsis Ca2+/H+ antiporter: identification of an N-terminal autoinhibitory domain. Plant Physiol. 2001;127:1020–1029. [PMC free article] [PubMed] [Google Scholar]
- Rogers EE, Eide DJ, Guerinot ML. Altered selectivity in an Arabidopsis metal transporter. Proc Natl Acad Sci USA. 2000;97:12356–12360. doi: 10.1073/pnas.210214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Wagner GJ. Cadmium transport across tonoplast of vesicles from oat roots. J Biol Chem. 1993;268:12297–12302. [PubMed] [Google Scholar]
- Schumaker KS, Sze H. Ca2+/H+ antiport system driven by the proton electrochemical gradient of a tonoplast H+-ATPase from oat roots. Plant Physiol. 1985;79:1111–1117. doi: 10.1104/pp.79.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul O, Hilgemann DW, de-Almeida-Engler J, Van Montagu M, Inzé D, Galili G. Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J. 1999;18:3973–3980. doi: 10.1093/emboj/18.14.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F, Fink GR, Hicks JB. Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- Shigaki T, Hirschi KD. Characterization of CAX-like genes in plants: implications for functional diversity. Gene. 2000;257:291–298. doi: 10.1016/s0378-1119(00)00390-5. [DOI] [PubMed] [Google Scholar]
- Shigaki T, Hirschi KD. Use of class IIS restriction enzymes for site-directed mutagenesis: variations on phoenix mutagenesis. Anal Biochem. 2001;298:118–120. doi: 10.1006/abio.2001.5341. [DOI] [PubMed] [Google Scholar]
- Shigaki T, Cheng NH, Pittman JK, Hirschi KD. Structural determinants of Ca2+ transport in the Arabidopsis H+/Ca2+ antiporter CAX1. J Biol Chem. 2001;276:43152–43159. doi: 10.1074/jbc.M106637200. [DOI] [PubMed] [Google Scholar]
- Stagljar I, Korostensky C, Johnsson N, Heesen ST. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc Natl Acad Sci USA. 1998;95:5187–5192. doi: 10.1073/pnas.95.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga SS, Imagawa S, Maeshima M. Specificity of the accumulation of mRNAs and proteins of the plasma membrane and tonoplast aquaporins in radish organs. Planta. 2001;212:294–304. doi: 10.1007/s004250000396. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighing, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas FM, Walton PE, Dunshea FR, Ballard FJ. IGF-I variants which bind poorly to IGF-binding proteins show more potent and prolonged hypoglycaemic action than native IGF-I in pigs and marmoset monkeys. J Endocrinol. 1997;155:377–386. doi: 10.1677/joe.0.1550377. [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi H, Nakanishi Y, Maeshima M. Properties and molecular cloning of Ca2+/H+ antiporter in the vacuolar membrane of mung bean. Eur J Biochem. 1999;262:417–425. doi: 10.1046/j.1432-1327.1999.00377.x. [DOI] [PubMed] [Google Scholar]
- Ueoka-Nakanishi H, Tsuchiya T, Sasaki M, Nakanishi Y, Cunningham KW, Maeshima M. Functional expression of mung bean Ca2+/H+ antiporter in yeast and its intracellular localization in the hypocotyl and tobacco cells. Eur J Biochem. 2000;267:3090–3098. doi: 10.1046/j.1432-1033.2000.01343.x. [DOI] [PubMed] [Google Scholar]
- Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989;58:409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]