Abstract
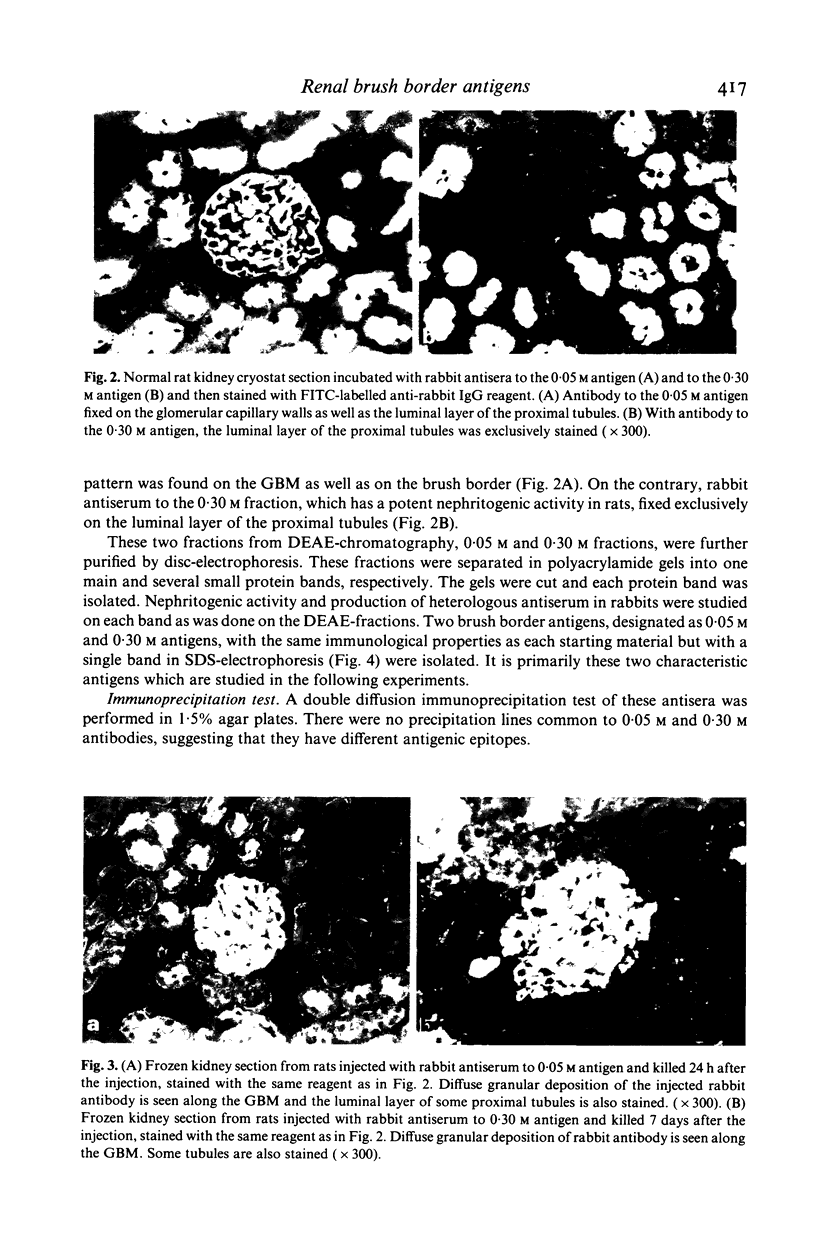
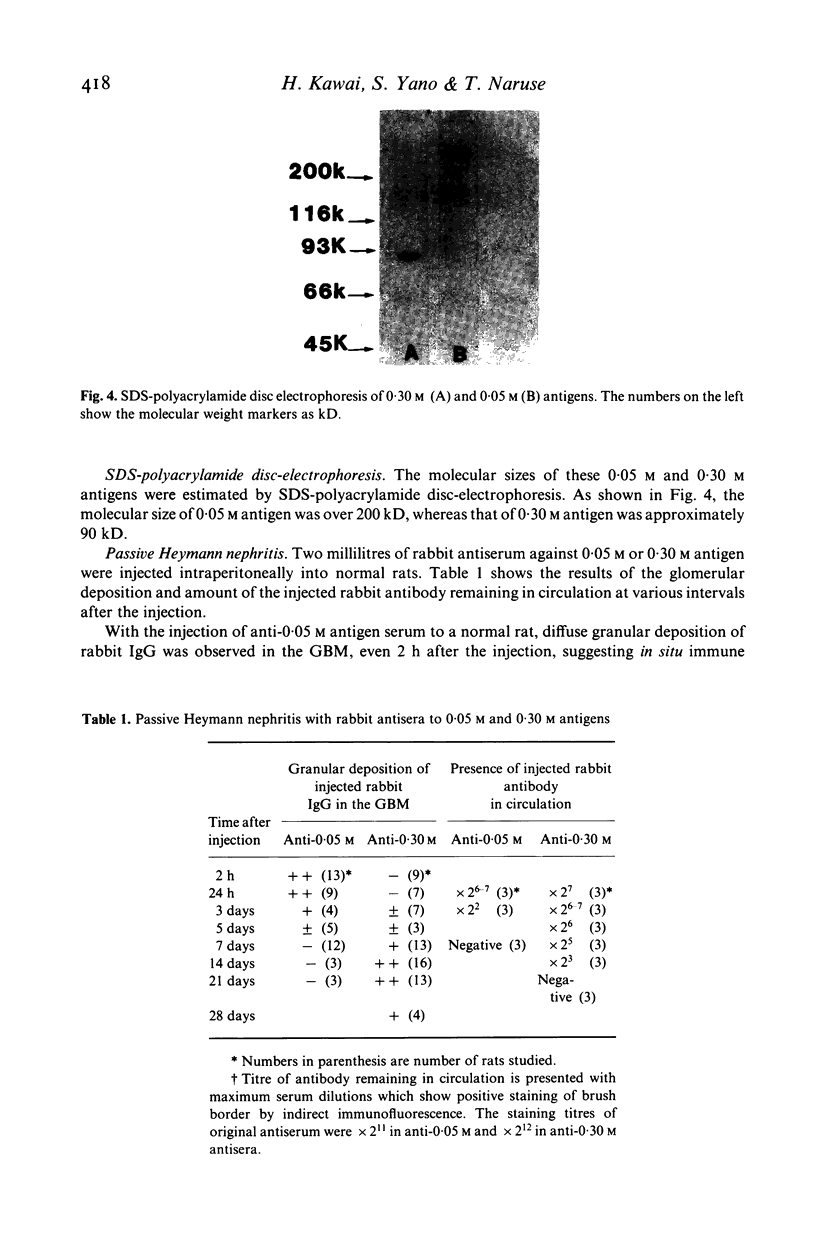
Two kinds of brush border antigens were isolated from pronase-treated rat tubular material by gel filtration, DEAE-chromatography and disc-electrophoresis, successively. One, a 0.05 M antigen which was eluted from DEAE-column with 0.05 mol of NaCl solution, has no nephritogenic ability when inoculated into homologous rats. The other, a 0.30 M antigen eluted with 0.30 mol of NaCl solution, induces membranous nephritis when injected into rats. Immunoprecipitation studies show no common factor between these two antigens. SDS-polyacrylamide electrophoresis shows the molecular size of 0.05 M and 0.30 M antigens to be respectively over 200 kD and about 90 kD. Rabbit antiserum against the 0.05 M antigen fixed to the GBM in a diffuse granular fashion as well as to the brush border by immunofluorescence when incubated in vitro with normal rat kidney section. Rabbit antiserum to the 0.30 M antigen, however, fixed exclusively in vitro to the brush border. Passive transfer of nephritis was studied with these rabbit antisera. When antiserum to 0.05 M antigen was injected into normal rat, diffuse granular deposition of rabbit IgG was observed in the GBM within 2 h of the injection, but the deposits became negative 1 week later. Rats injected with antiserum to the 0.30 M antigen showed no glomerular deposition within 2 days but diffuse granular deposits of rabbit IgG were observed within 1 week and increased until 2 weeks after the injection. These facts should be considered in the studies on passive Heymann nephritis and its pathogenesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barabas A. Z., Lannigan R. Induction of an autologous immune-complex glomerulonephritis in the rat by intravenous injection of heterologous anti-rat kidney tubular antibody. I. Production of chronic progressive immune-complex glomerulonephritis. Br J Exp Pathol. 1974 Feb;55(1):47–55. [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Douglas M. F., Rabideau D. P., Schwartz M. M., Lewis E. J. Evidence of autologous immune-complex nephritis. N Engl J Med. 1981 Nov 26;305(22):1326–1329. doi: 10.1056/NEJM198111263052206. [DOI] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. I. Identification and isolation of the pathogenetic antigen. J Exp Med. 1968 Mar 1;127(3):555–572. doi: 10.1084/jem.127.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Watson J. I., Dixon F. J. Characterization and isolation of specific renal tubular epithelial antigens. J Immunol. 1967 Dec;99(6):1199–1210. [PubMed] [Google Scholar]
- Fleuren G. J., Grond J., Hoedemaeker P. J. The pathogenetic role of free-circulating antibody in autologous immune complex glomerulonephritis. Clin Exp Immunol. 1980 Aug;41(2):205–217. [PMC free article] [PubMed] [Google Scholar]
- Fleuren G. J., vd Lee R., Greben H. A., Van Damme B. J., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. IV. Investigations into the pathogenesis of the model. Lab Invest. 1978 Apr;38(4):496–501. [PubMed] [Google Scholar]
- Grupe W. E., Kaplan M. H. Demonstration of an antibody to proximal tubular antigen in the pathogenesis of experimental autoimmune nephrosis in rats. J Lab Clin Med. 1969 Sep;74(3):400–409. [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaio M. P., Salant D. J., Cohen A. J., Adler S., Couser W. G. Comparative study of in situ immune deposit formation in active and passive Heymann nephritis. Kidney Int. 1983 Mar;23(3):498–505. doi: 10.1038/ki.1983.47. [DOI] [PubMed] [Google Scholar]
- Makker S. P., Moorthy B. In situ immune complex formation in isolated perfused kidney using homologous antibody. Lab Invest. 1981 Jan;44(1):1–5. [PubMed] [Google Scholar]
- Makker S. P., Singh A. K. Characterization of the antigen (gp600) of Heymann nephritis. Lab Invest. 1984 Mar;50(3):287–293. [PubMed] [Google Scholar]
- Miettinen A., Törnroth T., Tikkanen I., Virtanen I., Linder E. Heymann nephritis induced by kidney brush border glycoproteins. Lab Invest. 1980 Dec;43(6):547–555. [PubMed] [Google Scholar]
- Naruse T., Fukasawa T., Hirokawa N., Oike S., Miyakawa Y. The pathogenesis of experimental membranous glomerulonephritis induced with homologous nephritogenic tubular antigen. J Exp Med. 1976 Nov 2;144(5):1347–1362. doi: 10.1084/jem.144.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse T., Fukasawa T., Umegae S., Oike S., Miyakawa Y. Experimental membranous glomerulonephritis in rats: correlation of ultrastructural changes with the serum level of autologous antibody against tubular antigen. Lab Invest. 1978 Aug;39(2):120–127. [PubMed] [Google Scholar]
- Neale T. J., Wilson C. B. Glomerular antigens in Heymann's nephritis: reactivity of eluted and circulating antibody. J Immunol. 1982 Jan;128(1):323–330. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Ozawa T., Boedecker E. A., Schorr W., Guggenheim S., McIntosh R. M. Immune-complex disease with unilateral renal vein thrombosis. Arch Pathol Lab Med. 1976 May;100(5):279–282. [PubMed] [Google Scholar]
- Ronco P., Allegri L., Melcion C., Pirotsky E., Appay M. D., Bariety J., Pontillon F., Verroust P. A monoclonal antibody to brush border and passive Heymann nephritis. Clin Exp Immunol. 1984 Feb;55(2):319–332. [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Strauss J., Pardo V., Koss M. N., Griswold W., McIntosh R. M. Nephropathy associated with sickle cell anemia: an autologous immune complex nephritis. I. Studies on nature of glomerular-bound antibody and antigen identification in a patient with sickle cell disease and immune deposit glomerulonephritis. Am J Med. 1975 Mar;58(3):382–387. doi: 10.1016/0002-9343(75)90604-x. [DOI] [PubMed] [Google Scholar]
- Van Damme B. J., Fleuren G. J., Bakker W. W., Vernier R. L., Hoedemaeker P. J. Experimental glomerulonephritis in the rat induced by antibodies directed against tubular antigens. V. Fixed glomerular antigens in the pathogenesis of heterologous immune complex glomerulonephritis. Lab Invest. 1978 Apr;38(4):502–510. [PubMed] [Google Scholar]