Abstract
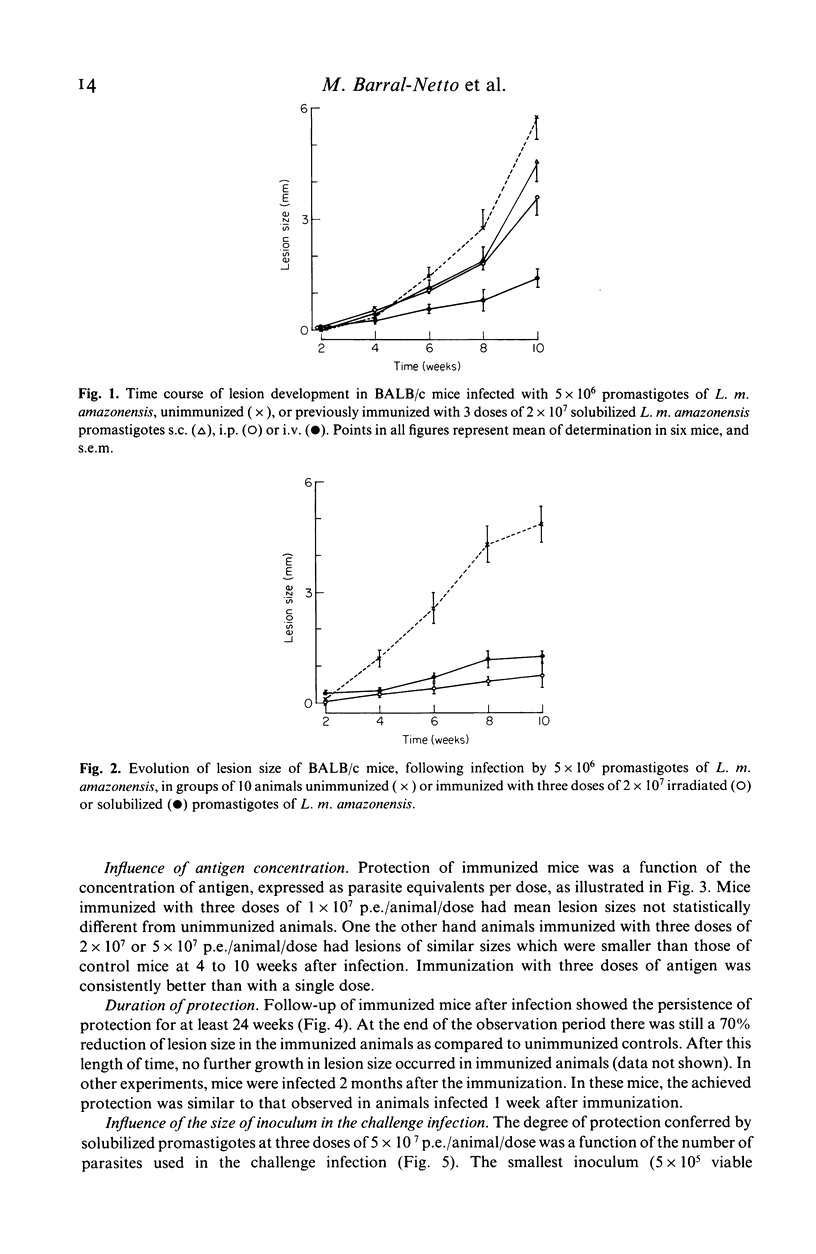
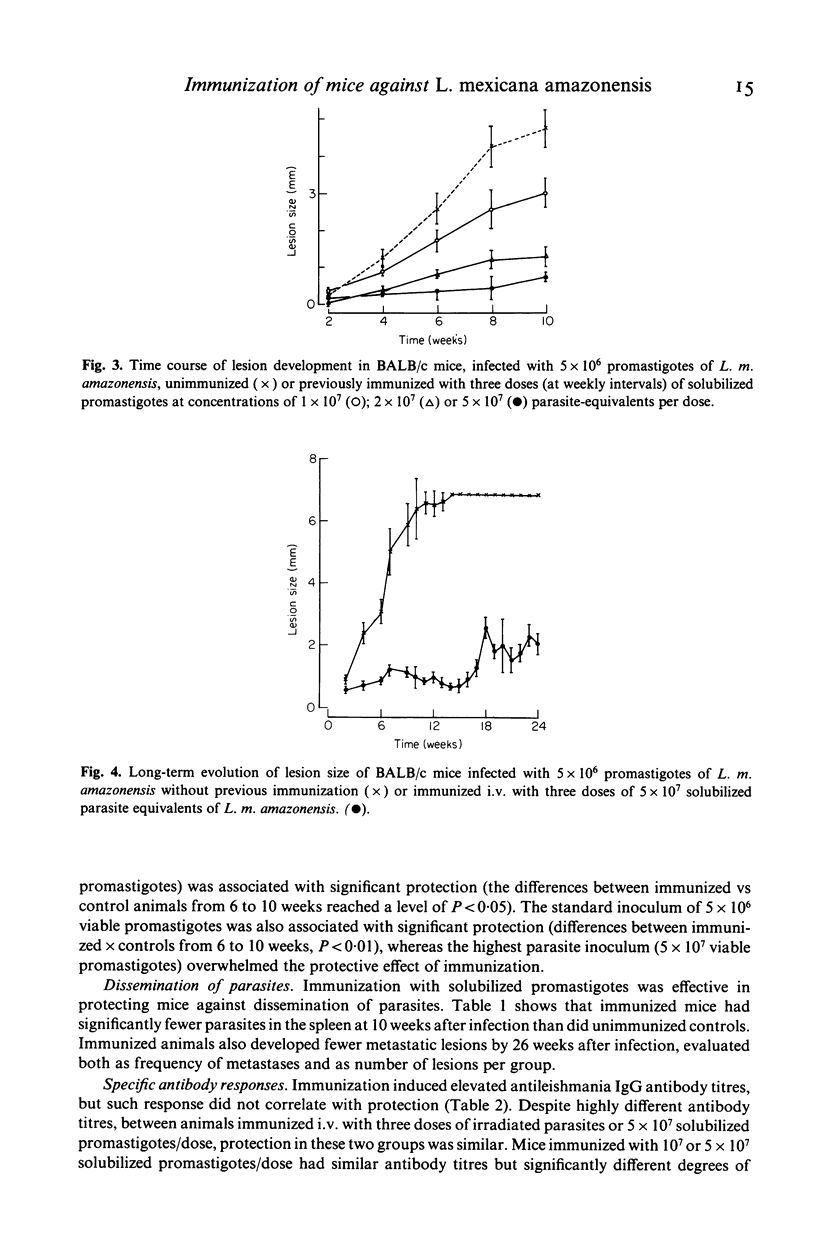
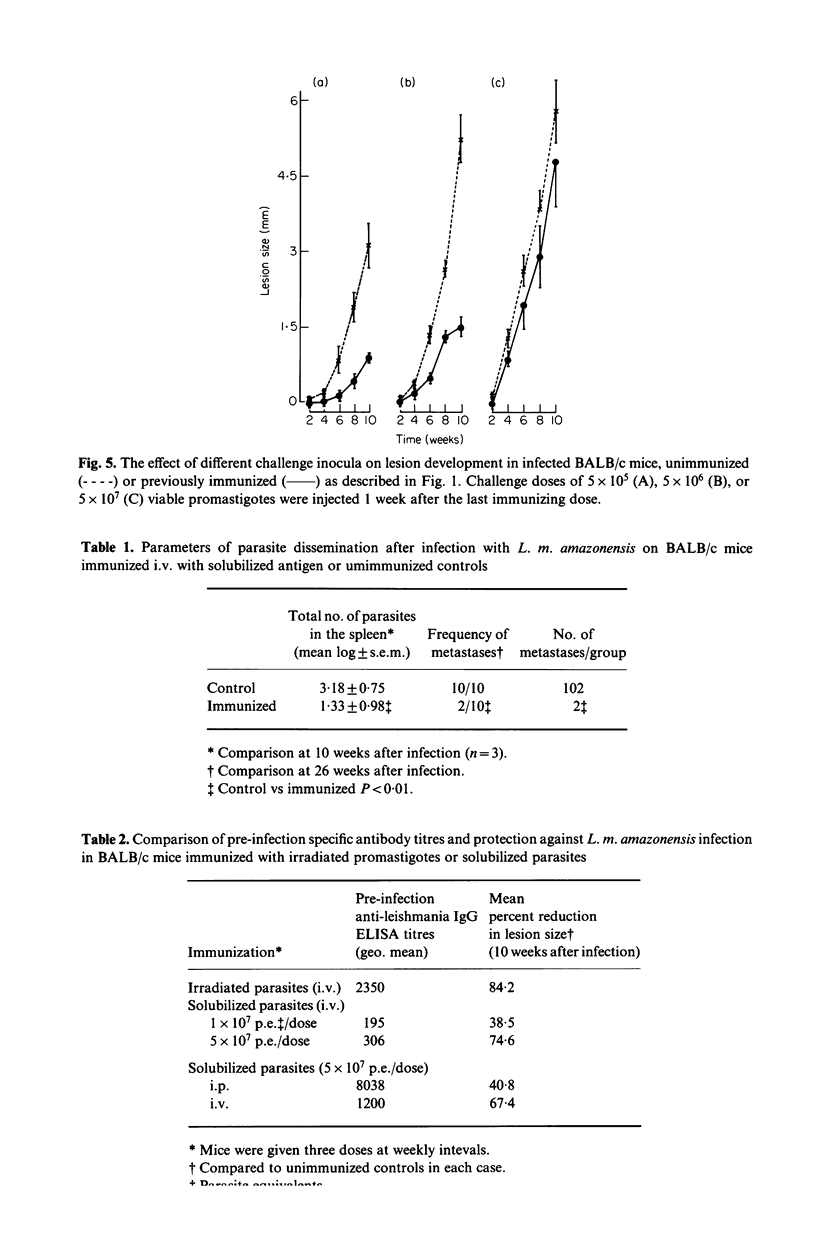
Successful immunization of highly susceptible BALB/c mice against progressive infection by Leishmania mexicana amazonensis, using whole solubilized promastigotes was achieved. The best immunization schedule consisted of three weekly injections of 5 X 10(7) parasite equivalents. Intravenous was superior to intraperitoneal or subcutaneous immunization. Protection persisted for up to 2 months after immunization, and beneficial effects could be observed in long-term follow-up (24 weeks after infection). Immunized mice exhibited marked reduction in primary lesion size, as well as reduction of the number of parasites in the spleen, and developed less metastases. High titres of specific anti-L. m. amazonensis IgG antibodies resulted from immunization, but titres did not correlate with protection. Groups with widely differing pre-infection antibody titres were equally protected, and similar antibody titres resulted in different levels of protection. Immunization alone did not induce significant serum interferon-gamma levels and specific delayed-type hypersensitivity (DTH) reactions, but resulted in the persistence of positive (DTH) reactions after infection, at a time when infected control animals had suppressed responses. Resistance to leishmaniasis appears to depend on cell mediated immune mechanisms, and the possibility of immunization with a solubilized antigen without adjuvant is intriguing and opens new perspectives in this area.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrade Z. A., Reed S. G., Roters S. B., Sadigursky M. Immunopathology of experimental cutaneous leishmaniasis. Am J Pathol. 1984 Jan;114(1):137–148. [PMC free article] [PubMed] [Google Scholar]
- Barral A., Petersen E. A., Sacks D. L., Neva F. A. Late metastatic Leishmaniasis in the mouse. A model for mucocutaneous disease. Am J Trop Med Hyg. 1983 Mar;32(2):277–285. doi: 10.4269/ajtmh.1983.32.277. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D., Bray R. S., Wolstencroft R. A., Dumonde D. C. Immunity in cutaneous leishmaniasis of the guinea-pig. Clin Exp Immunol. 1970 Sep;7(3):301–341. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D., Preston P. M., Bray R. S., Dumonde D. C. Experimental cutaneous leishmaniasis. II. Effects of immunosuppression and antigenic competition on the course of infection with Leishmania enriettii in the guinea-pig. Clin Exp Immunol. 1972 Feb;10(2):305–335. [PMC free article] [PubMed] [Google Scholar]
- DeTolla L. J., Scott P. A., Farrell J. P. Single gene control of resistance to cutaneous leishmaniasis in mice. Immunogenetics. 1981;14(1-2):29–39. doi: 10.1007/BF00344297. [DOI] [PubMed] [Google Scholar]
- Guirges S. Y. Natural and experimental re-infection of man with Oriental sore. Ann Trop Med Parasitol. 1971 Jun;65(2):197–205. doi: 10.1080/00034983.1971.11686746. [DOI] [PubMed] [Google Scholar]
- Hale C., Howard J. G. Immunological regulation of experimental cutaneous leishmaniasis. 2. Studies with Biozzi high and low responder lines of mice. Parasite Immunol. 1981 Spring;3(1):45–55. doi: 10.1111/j.1365-3024.1981.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980 Sep 1;152(3):594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Liew F. Y., Hale C., Nicklin S. Prophylactic immunization against experimental leishmaniasis. II. Further characterization of the protective immunity against fatal Leishmania tropica infection induced by irradiated promastigotes. J Immunol. 1984 Jan;132(1):450–455. [PubMed] [Google Scholar]
- Howard J. G., Nicklin S., Hale C., Liew F. Y. Prophylactic immunization against experimental leishmaniasis: I. Protection induced in mice genetically vulnerable to fatal Leishmania tropica infection. J Immunol. 1982 Nov;129(5):2206–2212. [PubMed] [Google Scholar]
- Milon G., Marchal G., Seman M., Truffa-Bachi P., Zilberfarb V. Is the delayed-type hypersensitivity observed after a low dose of antigen mediated by helper T cells? J Immunol. 1983 Mar;130(3):1103–1107. [PubMed] [Google Scholar]
- Mitchell G. F., Curtis J. M., Handman E. Resistance to cutaneous leishmaniasis in genetically susceptible BALB/c mice. Aust J Exp Biol Med Sci. 1981 Oct;59(Pt 5):555–565. doi: 10.1038/icb.1981.48. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. Leishmania tropica major in mice: vaccination against cutaneous leishmaniasis in mice of high genetic susceptibility. Aust J Exp Biol Med Sci. 1983 Feb;61(Pt 1):11–25. doi: 10.1038/icb.1983.2. [DOI] [PubMed] [Google Scholar]
- Mosser D. M., Edelson P. J. Activation of the alternative complement pathway by Leishmania promastigotes: parasite lysis and attachment to macrophages. J Immunol. 1984 Mar;132(3):1501–1505. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T. Mechanism of lethal effect of human serum upon Leishmania donovani. J Immunol. 1980 Nov;125(5):2195–2201. [PubMed] [Google Scholar]
- Preston P. M., Dumonde D. C. Experimental cutaneous leishmaniasis. V. Protective immunity in subclinical and self-healing infection in the mouse. Clin Exp Immunol. 1976 Jan;23(1):126–138. [PMC free article] [PubMed] [Google Scholar]
- Pérez H., Arredondo B., González M. Comparative study of American cutaneous leishmaniasis and diffuse cutaneous leishmaniasis in two strains of inbred mice. Infect Immun. 1978 Nov;22(2):301–307. doi: 10.1128/iai.22.2.301-307.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Lima G. C., Engers H. D., Louis J. A. Exacerbation of murine cutaneous leishmaniasis by adoptive transfer of parasite-specific helper T cell populations capable of mediating Leishmania major-specific delayed-type hypersensitivity. J Immunol. 1984 Sep;133(3):1594–1600. [PubMed] [Google Scholar]


