Abstract
Mononuclear cells were isolated from the inflamed muscle tissue of a patient suffering from dermatomyositis (DM). These were expanded in long-term culture and maintained in the presence of IL-2 containing culture medium. Two cell lines were established, one of the helper/inducer (OKT4+) and the other of the suppressor/cytotoxic phenotype (OKT8+). The OKT4+ cell line exhibited a non HLA-restricted, tissue-specific, myocytotoxic effect on rat muscle cell culture. Its lymphoproliferative response to human muscle antigen was HLA-restricted. The OKT8+ cell line exhibited a non HLA-restricted, tissue-specific response to muscle antigens and no myocytotoxic activity in in vitro rat muscle cell culture. It is likely that clones of OKT4+ lymphocytes in patients suffering from DM are associated with the pathogenesis of the disease--they probably mediate the diffuse damage to skeletal muscle through their myocytotoxic activity.
Full text
PDF





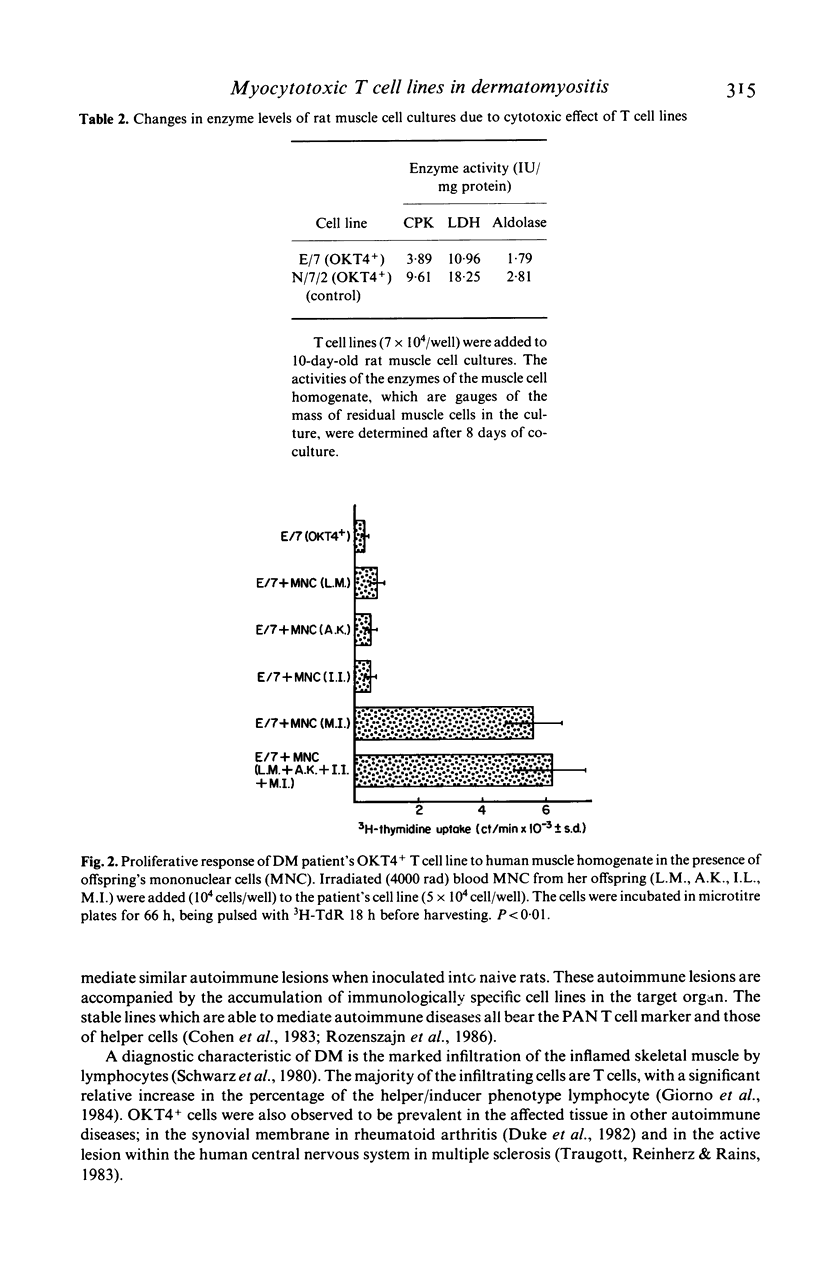
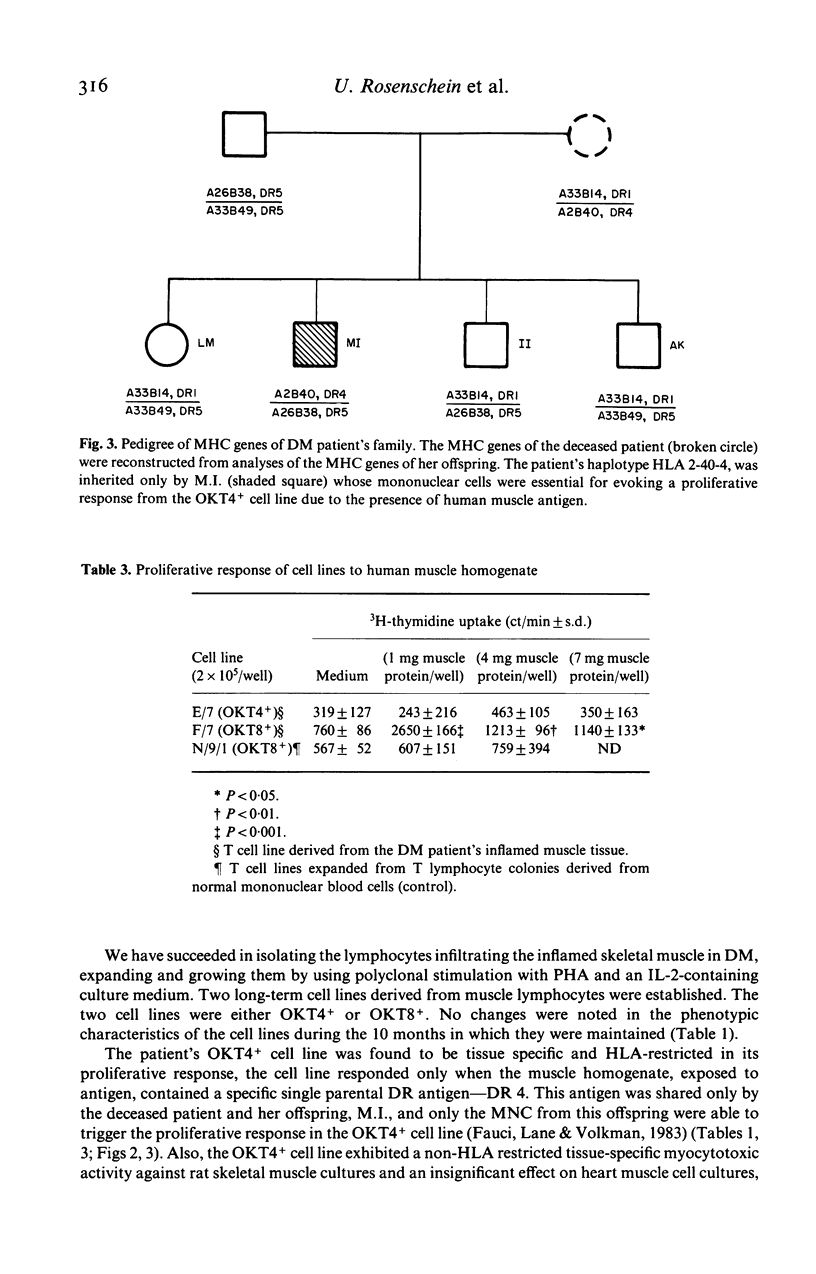


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohan A., Peter J. B. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975 Feb 13;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- Caspi R. R., Roberge F. G., McAllister C. G., el-Saied M., Kuwabara T., Gery I., Hanna E., Nussenblatt R. B. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986 Feb 1;136(3):928–933. [PubMed] [Google Scholar]
- Cohen A., Buckingham M., Gros F. A modified assay procedure for revealing the M form of creatine kinase in cultured muscle cells. Exp Cell Res. 1978 Aug;115(1):201–206. doi: 10.1016/0014-4827(78)90417-2. [DOI] [PubMed] [Google Scholar]
- Dawkins R. L., Mastaglia F. L. Cell-mediated cytotoxicity to muscle in polymyositis. Effect of immunosuppression. N Engl J Med. 1973 Mar 1;288(9):434–438. doi: 10.1056/NEJM197303012880903. [DOI] [PubMed] [Google Scholar]
- Duke O., Panayi G. S., Janossy G., Poulter L. W. An immunohistological analysis of lymphocyte subpopulations and their microenvironment in the synovial membranes of patients with rheumatoid arthritis using monoclonal antibodies. Clin Exp Immunol. 1982 Jul;49(1):22–30. [PMC free article] [PubMed] [Google Scholar]
- Garlepp M. J., Dawkins R. L. Inflammatory disorders of muscle. Immunological aspects. Clin Rheum Dis. 1984 Apr;10(1):35–51. [PubMed] [Google Scholar]
- Giorno R., Barden M. T., Kohler P. F., Ringel S. P. Immunohistochemical characterization of the mononuclear cells infiltrating muscle of patients with inflammatory and noninflammatory myopathies. Clin Immunol Immunopathol. 1984 Mar;30(3):405–412. doi: 10.1016/0090-1229(84)90026-6. [DOI] [PubMed] [Google Scholar]
- HARARY I., FARLEY B. In vitro studies on single beating rat heart cells. I. Growth and organization. Exp Cell Res. 1963 Feb;29:451–465. doi: 10.1016/s0014-4827(63)80008-7. [DOI] [PubMed] [Google Scholar]
- Haas D. C. Absence of cell-mediated cytotoxicity to muscle cultures in polymyositis. J Rheumatol. 1980 Sep-Oct;7(5):671–676. [PubMed] [Google Scholar]
- Janossy G., Tidman N., Papageorgiou E. S., Kung P. C., Goldstein G. Distribution of t lymphocyte subsets in the human bone marrow and thymus: an analysis with monoclonal antibodies. J Immunol. 1981 Apr;126(4):1608–1613. [PubMed] [Google Scholar]
- Kakulas B. A. In vitro destruction of skeletal muscle by sensitized cells. Nature. 1966 Jun 11;210(5041):1115–1118. doi: 10.1038/2101115a0. [DOI] [PubMed] [Google Scholar]
- MEULEMANS O. Determination of total protein in spinal fluid with sulphosalicylic acid and trichloroacetic acid. Clin Chim Acta. 1960 Sep;5:757–761. doi: 10.1016/0009-8981(60)90020-6. [DOI] [PubMed] [Google Scholar]
- Martonosi A., Roufa D., Boland R., Reyes E., Tillack T. W. Development of sarcoplasmic reticulum in cultured chicken muscle. J Biol Chem. 1977 Jan 10;252(1):318–332. [PubMed] [Google Scholar]
- Mastaglia F. L., Dawkins R. L., Papadimitriou J. M. Lymphocyte-muscle cell interactions in vivo and in vitro. J Neurol Sci. 1974 Jun;22(2):261–268. doi: 10.1016/0022-510x(74)90250-0. [DOI] [PubMed] [Google Scholar]
- Moretta A., Pantaleo G., Maggi E., Mingari M. C. Recent advances in the phenotypic and functional analysis of human T lymphocytes. Semin Hematol. 1984 Oct;21(4):257–269. [PubMed] [Google Scholar]
- Morgan G., Peter J. B., Newbould B. B. Experimental allergic myositis in rats. Arthritis Rheum. 1971 Sep-Oct;14(5):599–609. doi: 10.1002/art.1780140507. [DOI] [PubMed] [Google Scholar]
- Nussenblatt R. B., Palestine A. G., El-Saied M., Meyers S., Lando Z., Mullenberg C., Rozenszajn L. A. Long-term antigen specific and non-specific T-cell lines and clones in uveitis. Curr Eye Res. 1984 Feb;3(2):299–305. doi: 10.3109/02713688408997213. [DOI] [PubMed] [Google Scholar]
- Rosalki S. B. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967 Apr;69(4):696–705. [PubMed] [Google Scholar]
- Rozenszajn L. A., Goldman I., Kalechman Y., Michlin H., Sredni B., Zeevi A., Shoham D. T-lymphocyte colony growth in vitro: factors modulating clonal expansion. Immunol Rev. 1981;54:157–186. doi: 10.1111/j.1600-065x.1981.tb00437.x. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Schwarz H. A., Slavin G., Ward P., Ansell B. M. Muscle biopsy in polymyositis and dermatomyositis: a clinicopathological study. Ann Rheum Dis. 1980 Oct;39(5):500–507. doi: 10.1136/ard.39.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shainberg A., Brik H., Bar-Shavit R., Sampson S. R. Inhibition of acetylcholine receptor synthesis by thyroid hormones. J Endocrinol. 1984 May;101(2):141–147. doi: 10.1677/joe.0.1010141. [DOI] [PubMed] [Google Scholar]
- Shainberg A., Yagil G., Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971 May;25(1):1–29. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- Traugott U., Reinherz E. L., Raine C. S. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983 Jan 21;219(4582):308–310. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- Witemeyer S., Bankhurst A. D., Williams R. C., Jr A population of human cord blood lymphocytes which generates Fc receptors in vitro. Cell Immunol. 1977 Apr;30(1):54–65. doi: 10.1016/0008-8749(77)90047-8. [DOI] [PubMed] [Google Scholar]
- Wybran J., Carr M. C., Fudenberg H. H. The human rosette-forming cell as a marker of a population of thymus-derived cells. J Clin Invest. 1972 Oct;51(10):2537–2543. doi: 10.1172/JCI107069. [DOI] [PMC free article] [PubMed] [Google Scholar]



