Abstract
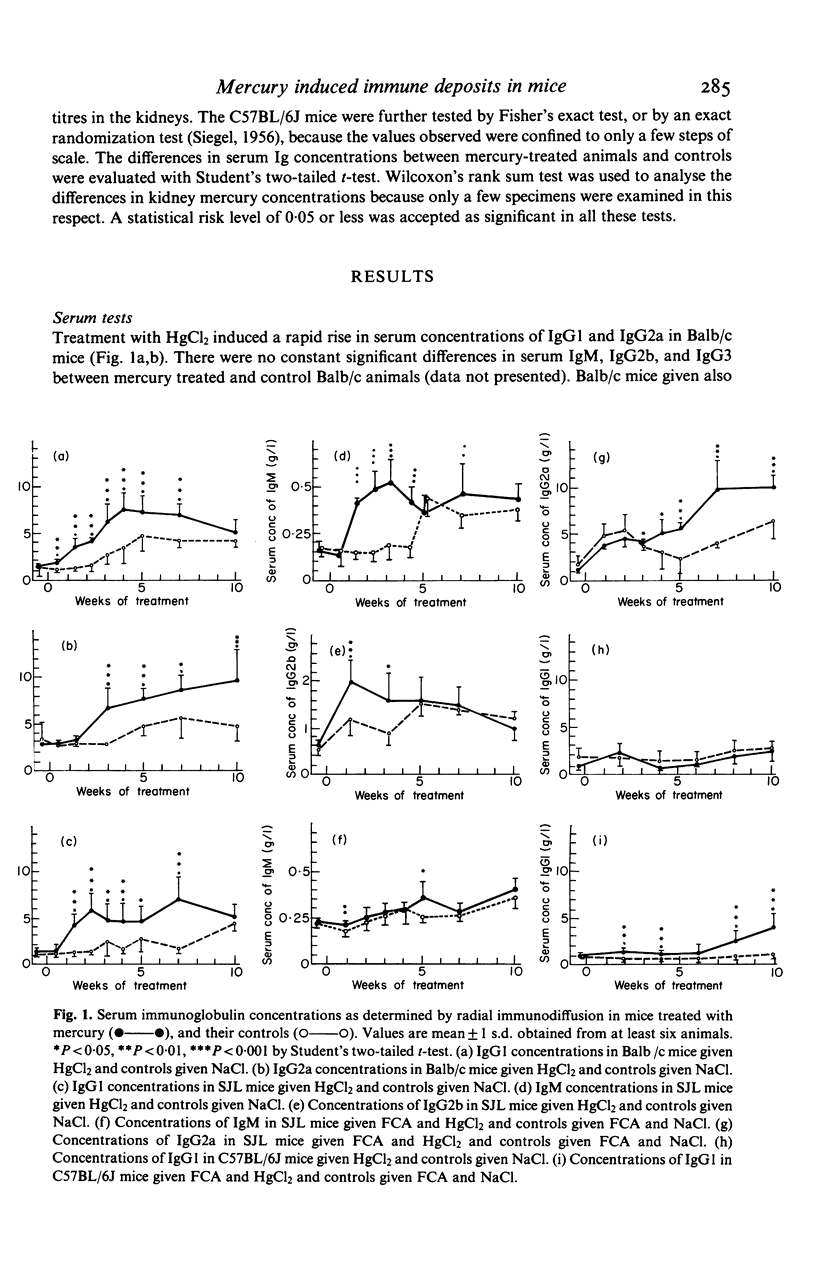
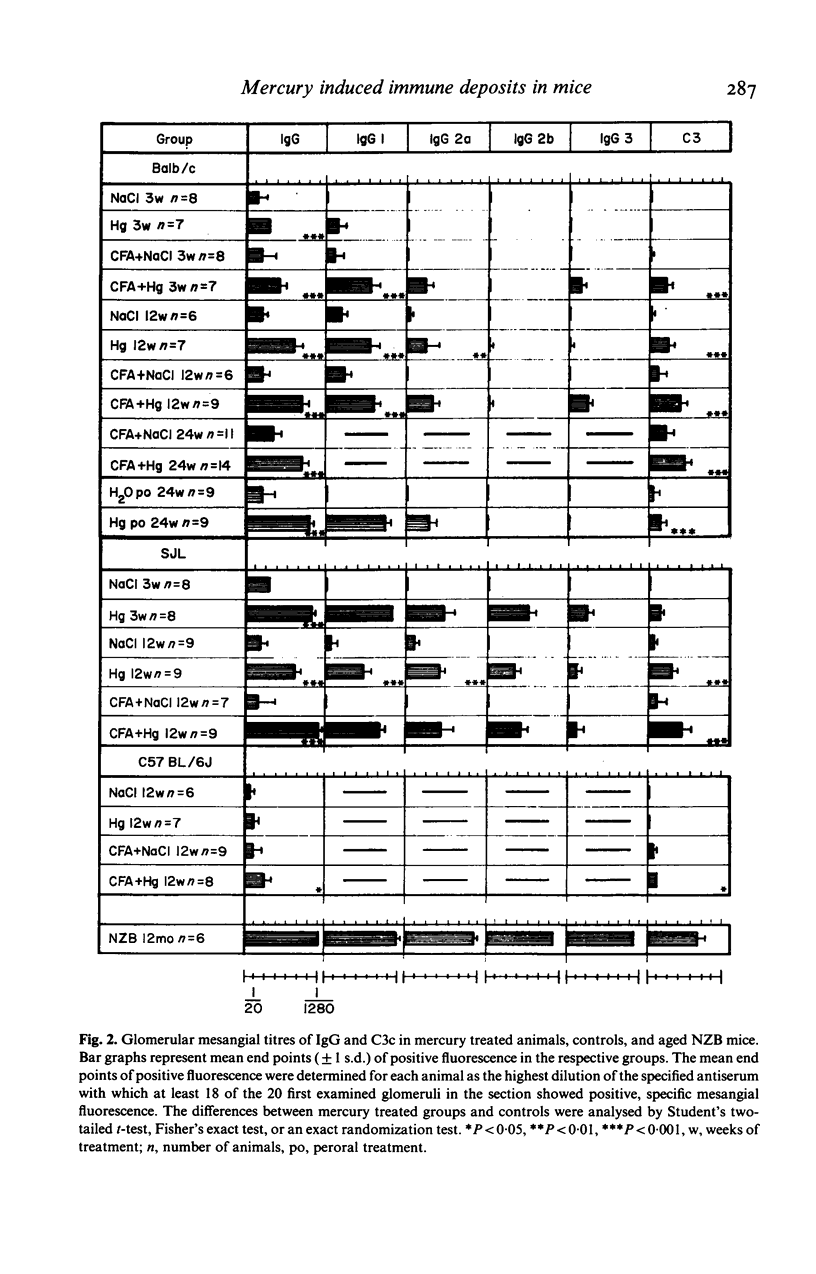
Female Balb/c and SJL mice exposed to HgCl2 by subcutaneous injection or via drinking water for up to 6 months showed immunostimulation with increased concentrations of immunoglobulins (Ig) in the serum. The Ig isotype pattern was dependent both on the strain and the use of immunopotentiation by addition of Freund's complete adjuvant (FCA). Balb/c mice showed a T cell dependent pattern, whereas the SJL mice developed an increase also in T cell independent isotypes. This latter pattern shifted to show T cell dependency after an initial addition of FCA. FCA also converted the lack of stimulation in C57BL/6J mice to a low response with a T cell dependent isotype pattern. No correlation emerged between the body burden of mercury, as assessed by the concentration in the kidneys, and the degree of immunostimulation by mercury. Mice showing a stimulation of the immune system developed mesangial immune complex (IC) glomerulonephritis and, later on, IC deposits in renal, splenic, and hepatic vessel walls with an isotype pattern corresponding to that seen in the serum.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accinni L., Dixon F. J. Degenerative vascular disease and myocardial infarction in mice with lupus-like syndrome. Am J Pathol. 1979 Aug;96(2):477–492. [PMC free article] [PubMed] [Google Scholar]
- Andrews B. S., Eisenberg R. A., Theofilopoulos A. N., Izui S., Wilson C. B., McConahey P. J., Murphy E. D., Roths J. B., Dixon F. J. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978 Nov 1;148(5):1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin A. A., Coutinho A. Specific T helper cells that activate B cells polyclonally. In vitro enrichment and cooperative function. J Exp Med. 1980 Mar 1;151(3):587–601. doi: 10.1084/jem.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellon B., Capron M., Druet E., Verroust P., Vial M. C., Sapin C., Girard J. F., Foidart J. M., Mahieu P., Druet P. Mercuric chloride induced autoimmune disease in Brown-Norway rats: sequential search for anti-basement membrane antibodies and circulating immune complexes. Eur J Clin Invest. 1982 Apr;12(2):127–133. doi: 10.1111/j.1365-2362.1982.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Bernaudin J. F., Druet E., Belair M. F., Pinchon M. C., Sapin C., Druet P. Extrarenal immune complex type deposits induced by mercuric chloride in the Brown Norway rat. Clin Exp Immunol. 1979 Nov;38(2):265–273. [PMC free article] [PubMed] [Google Scholar]
- Bernaudin J. F., Druet E., Druet P., Masse R. Inhalation or ingestion of organic or inorganic mercurials produces auto-immune disease in rats. Clin Immunol Immunopathol. 1981 Jul;20(1):129–135. doi: 10.1016/0090-1229(81)90170-7. [DOI] [PubMed] [Google Scholar]
- Blakley B. R., Sisodia C. S., Mukkur T. K. The effect of methylmercury, tetraethyl lead, and sodium arsenite on the humoral immune response in mice. Toxicol Appl Pharmacol. 1980 Feb;52(2):245–254. doi: 10.1016/0041-008x(80)90111-8. [DOI] [PubMed] [Google Scholar]
- Bourcier D. R., Sharma R. P. A stationary cold-vapor technique for the determination of submicrogram amounts of mercury in biological tissues by flameless atomic absorption spectrophotometry. J Anal Toxicol. 1981 Mar-Apr;5(2):65–68. doi: 10.1093/jat/5.2.65. [DOI] [PubMed] [Google Scholar]
- Brentjens J. R., O'Connell D. W., Albini B., Andres G. A. Experimental chronic serum sickness in rabbits that received daily multiple and high doses of antigen: a systemic disease. Ann N Y Acad Sci. 1975 Jun 30;254:603–613. doi: 10.1111/j.1749-6632.1975.tb29207.x. [DOI] [PubMed] [Google Scholar]
- Dieter M. P., Luster M. I., Boorman G. A., Jameson C. W., Dean J. H., Cox J. W. Immunological and biochemical responses in mice treated with mercuric chloride. Toxicol Appl Pharmacol. 1983 Apr;68(2):218–228. doi: 10.1016/0041-008x(83)90006-6. [DOI] [PubMed] [Google Scholar]
- Druet E., Sapin C., Fournie G., Mandet C., Günther E., Druet P. Genetic control of susceptibility to mercury-induced immune nephritis in various strains of rat. Clin Immunol Immunopathol. 1982 Nov;25(2):203–212. doi: 10.1016/0090-1229(82)90183-0. [DOI] [PubMed] [Google Scholar]
- Druet E., Sapin C., Günther E., Feingold N., Druet P. Mercuric chloride-induced anti-glomerular basement membrane antibodies in the rat: genetic control. Eur J Immunol. 1977 Jun;7(6):348–351. doi: 10.1002/eji.1830070605. [DOI] [PubMed] [Google Scholar]
- Druet P., Druet E., Potdevin F., Sapin C. Immune type glomerulonephritis induced by HgCl2 in the Brown Norway rat. Ann Immunol (Paris) 1978 Oct-Dec;129 100(6):777–792. [PubMed] [Google Scholar]
- Eneström S., Hultman P. Immune-mediated glomerulonephritis induced by mercuric chloride in mice. Experientia. 1984 Nov 15;40(11):1234–1240. doi: 10.1007/BF01946653. [DOI] [PubMed] [Google Scholar]
- Forni L. From the point of view of the B cell: considerations on B-cell activation. Scand J Immunol. 1985 Sep;22(3):235–243. doi: 10.1111/j.1365-3083.1985.tb01876.x. [DOI] [PubMed] [Google Scholar]
- Gaworski C. L., Sharma R. P. The effects of heavy metals on [3H]thymidine uptake in lymphocytes. Toxicol Appl Pharmacol. 1978 Nov;46(2):305–313. doi: 10.1016/0041-008x(78)90076-5. [DOI] [PubMed] [Google Scholar]
- Gerstner H. B., Huff J. E. Clinical toxicology of mercury. J Toxicol Environ Health. 1977 Jan;2(3):491–526. doi: 10.1080/15287397709529452. [DOI] [PubMed] [Google Scholar]
- Gronowicz E. S., Doss C., Assisi F., Vitetta E. S., Coffman R. L., Strober S. Surface Ig isotypes on cells responding to lipopolysaccharide by IgM and IgG secretion. J Immunol. 1979 Nov;123(5):2049–2056. [PubMed] [Google Scholar]
- Hadjipetrou-Kourounakis L., Möller E. Adjuvants influence the immunoglobin subclass distribution of immune responses in vivo. Scand J Immunol. 1984 Mar;19(3):219–225. doi: 10.1111/j.1365-3083.1984.tb00923.x. [DOI] [PubMed] [Google Scholar]
- Hirsch F., Couderc J., Sapin C., Fournie G., Druet P. Polyclonal effect of HgCl2 in the rat, its possible role in an experimental autoimmune disease. Eur J Immunol. 1982 Jul;12(7):620–625. doi: 10.1002/eji.1830120716. [DOI] [PubMed] [Google Scholar]
- Hultman P., Eneström S., von Schenck H. Renal handling of inorganic mercury in mice. The early excretion phase following a single intravenous injection of mercuric chloride studied by the Silver Amplification method. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49(3):209–224. [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. Subclass-restricted IgG polyclonal antibody production in mice injected with lipid A-rich lipopolysaccharides. J Exp Med. 1981 Feb 1;153(2):324–338. doi: 10.1084/jem.153.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence D. A. Heavy metal modulation of lymphocyte activities. 1. In vitro effects of heavy metals on primary humoral immune responses. Toxicol Appl Pharmacol. 1981 Mar 15;57(3):439–451. doi: 10.1016/0041-008x(81)90241-6. [DOI] [PubMed] [Google Scholar]
- Makker S. P., Aikawa M. Mesangial glomerulonephropathy with deposition of IgG, IgM, and C3 induced by mercuric chloride: a new model. Lab Invest. 1979 Jul;41(1):45–50. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Martinez-Alonso C., Coutinho A., Augustin A. A. Immunoglobulin C-gene expression. I. The commitment to IgG subclass of secretory cells is determined by the quality of the nonspecific stimuli. Eur J Immunol. 1980 Sep;10(9):698–702. doi: 10.1002/eji.1830100908. [DOI] [PubMed] [Google Scholar]
- Nakashima S., Kamikawa H. Accelerated expansion of antibody heterogeneity by complete Freund's adjuvant during the response to bacterial alpha-amylase. Immunology. 1984 Dec;53(4):837–845. [PMC free article] [PubMed] [Google Scholar]
- Ohi G., Fukuda M., Seto H., Yagyu H. Effect of methylmercury on humoral immune responses in mice under conditions simulated to practical situations. Bull Environ Contam Toxicol. 1976 Feb;15(2):175–180. doi: 10.1007/BF01685157. [DOI] [PubMed] [Google Scholar]
- Perlmutter R. M., Hansburg D., Briles D. E., Nicolotti R. A., Davie J. M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978 Aug;121(2):566–572. [PubMed] [Google Scholar]
- Robinson C. J., Abraham A. A., Balazs T. Induction of anti-nuclear antibodies by mercuric chloride in mice. Clin Exp Immunol. 1984 Nov;58(2):300–306. [PMC free article] [PubMed] [Google Scholar]
- Roman-Franco A. A., Turiello M., Albini B., Ossi E., Milgrom F., Andres G. A. Anti-basement membrane antibodies and antigen--antibody complexes in rabbits injected with mercuric chloride. Clin Immunol Immunopathol. 1978 Apr;9(4):464–481. doi: 10.1016/0090-1229(78)90143-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg Y. J. Isotype-specific T cell regulation of immunoglobulin expression. Immunol Rev. 1982;67:33–58. doi: 10.1111/j.1600-065x.1982.tb01054.x. [DOI] [PubMed] [Google Scholar]
- Sapin C., Druet E., Druet P. Induction of anti-glomerular basement membrane antibodies in the Brown-Norway rat by mercuric chloride. Clin Exp Immunol. 1977 Apr;28(1):173–179. [PMC free article] [PubMed] [Google Scholar]
- Slack J. H., Hang L., Barkley J., Fulton R. J., D'Hoostelaere L., Robinson A., Dixon F. J. Isotypes of spontaneous and mitogen-induced autoantibodies in SLE-prone mice. J Immunol. 1984 Mar;132(3):1271–1275. [PubMed] [Google Scholar]
- Slack J., Der-Balian G. P., Nahm M., Davie J. M. Subclass restriction of murine antibodies. II. The IgG plaque-forming cell response to thymus-independent type 1 and type 2 antigens in normal mice and mice expressing an X-linked immunodeficiency. J Exp Med. 1980 Apr 1;151(4):853–862. doi: 10.1084/jem.151.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening J. J., Fleuren G. J., Hoedemaeker P. J. Demonstration of antinuclear antibodies in mercuric chloride-induced glomerulopathy in the rat. Lab Invest. 1978 Oct;39(4):405–411. [PubMed] [Google Scholar]
- Weening J. J., Grond J., van der Top D., Hoedemaeker P. J. Identification of the nuclear antigen involved in mercury-induced glomerulopathy in the rat. Invest Cell Pathol. 1980 Apr-Jun;3(2):129–134. [PubMed] [Google Scholar]