Abstract
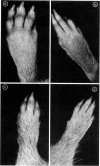
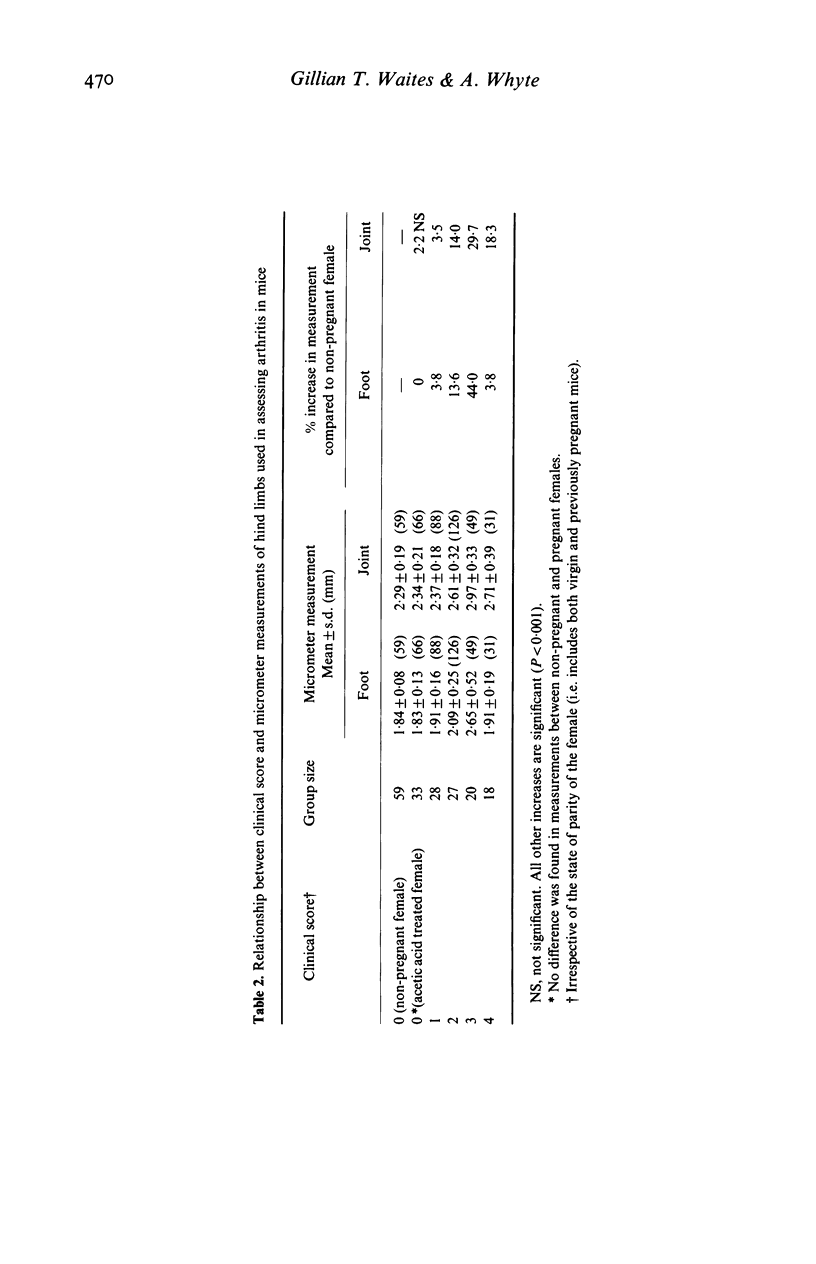
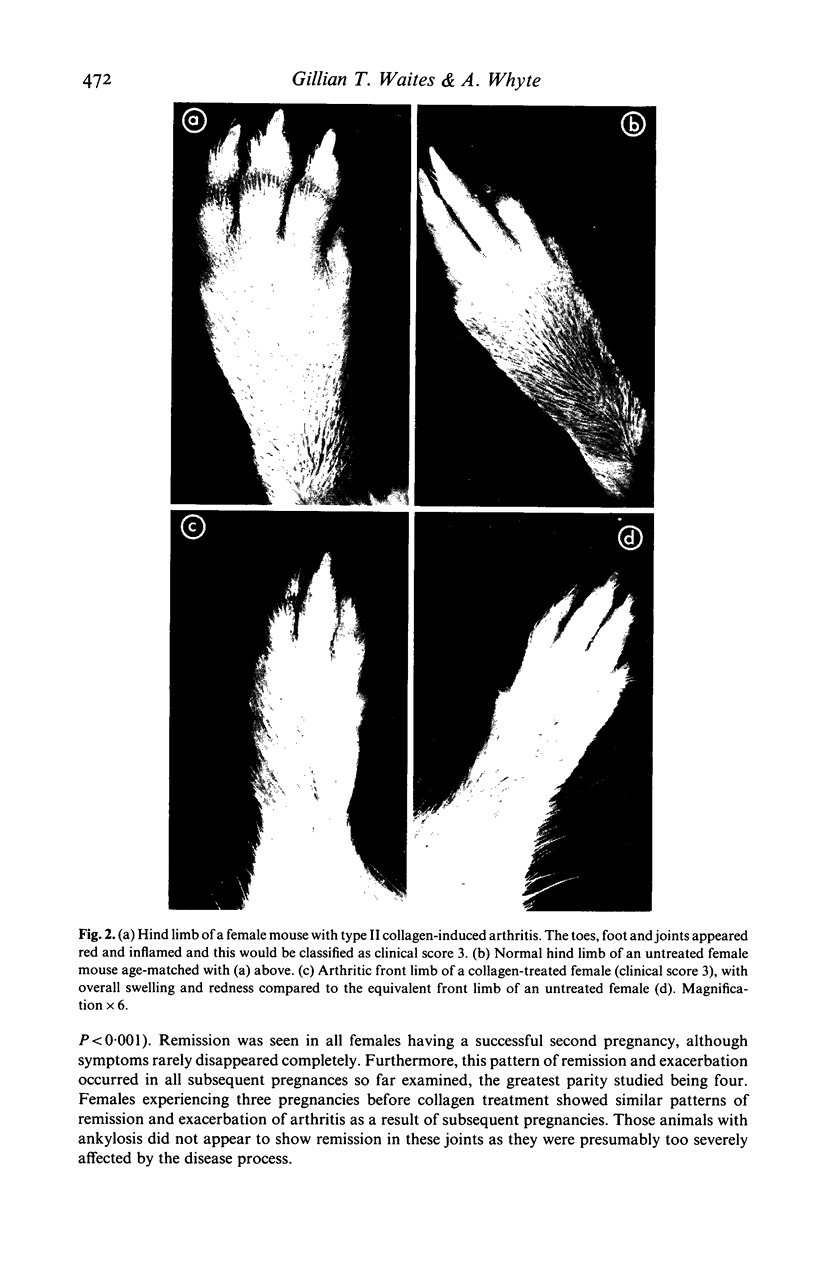
Bovine type II collagen administered to non-pregnant female DBA/1 mice caused arthritis in 55% of animals with a mean onset time of 70 days following immunization. Of collagen-treated females subsequently becoming syngeneically pregnant before the onset of arthritis, all developed the disease within 10 days of parturition, representing an earlier onset, compared to non-pregnant females, of 41 days. This earlier onset also occurred in females with an allogenic pregnancy, but did not occur in females resorbing their fetuses (only syngeneic pregnancies were examined). In females with arthritis at the time of conception a significant remission was observed during pregnancy followed by exacerbation post-partum. This sequence of remissions during pregnancy and exacerbations post-partum occurred with each pregnancy (parties of up to four studied). The treatment of multiparous females with collagen demonstrated that pregnancy does not provide long-term protection against the development or progression of arthritis, as such females were equally susceptible to post-partum onset of collagen-induced arthritis (CIA) and the remissions and exacerbations described above. The modifying effect of pregnancy on CIA in mice is complex and does not appear to be the result of a single pregnancy-associated phenomenon.
Full text
PDF


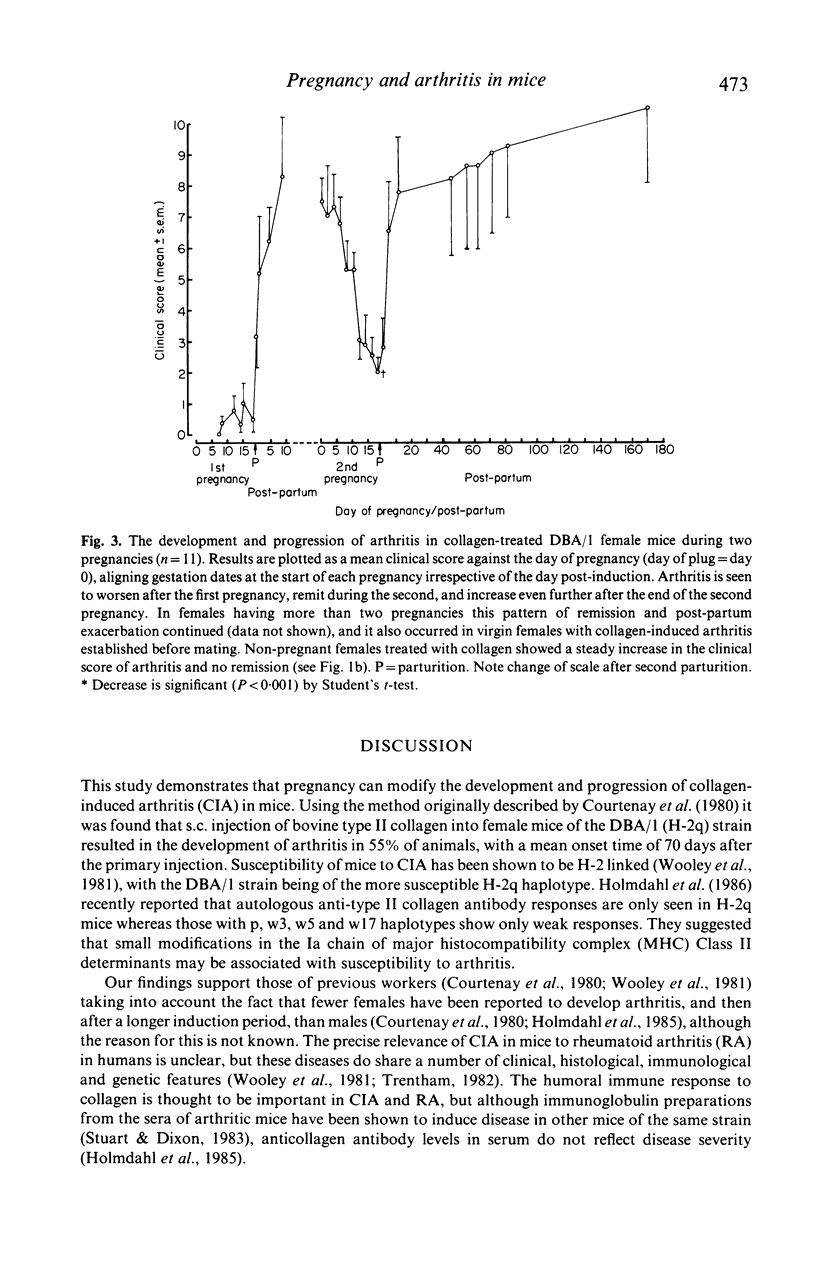



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelboom T., Persellin R. H. Leukocyte sensitization against synovial components in rheumatoid arthritis: blocking by pregnancy serum. Int Arch Allergy Appl Immunol. 1984;74(3):200–205. doi: 10.1159/000233543. [DOI] [PubMed] [Google Scholar]
- Carter S. D., Bourne J. T., Elson C. J., Hutton C. W., Czudek R., Dieppe P. A. Mononuclear phagocytes in rheumatoid arthritis: Fc-receptor expression by peripheral blood monocytes. Ann Rheum Dis. 1984 Jun;43(3):424–429. doi: 10.1136/ard.43.3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay J. S., Dallman M. J., Dayan A. D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980 Feb 14;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Harrison M. R. Maternal immunocompetence. II. Proliferative responses of maternal lymphocytes in vitro and inhibition by serum from pregnant rats. Scand J Immunol. 1976;5(8):881–889. doi: 10.1111/j.1365-3083.1976.tb03038.x. [DOI] [PubMed] [Google Scholar]
- Herbage D., Bouillet J., Bernengo J. C. Biochemical and physiochemical characterization of pepsin-solubilized type-II collagen from bovine articular cartilage. Biochem J. 1977 Feb 1;161(2):303–312. doi: 10.1042/bj1610303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahara F., Wooley P. H., Luthra H. S., Coulam C. B., Griffiths M. M., David C. S. Collagen-induced arthritis and pregnancy in mice: the effects of pregnancy on collagen-induced arthritis and the high incidence of infertility in arthritic female mice. Am J Reprod Immunol Microbiol. 1986 Jun;11(2):44–54. doi: 10.1111/j.1600-0897.1986.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Holmdahl R., Jansson L., Gullberg D., Rubin K., Forsberg P. O., Klareskog L. Incidence of arthritis and autoreactivity of anti-collagen antibodies after immunization of DBA/1 mice with heterologous and autologous collagen II. Clin Exp Immunol. 1985 Dec;62(3):639–646. [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R., Klareskog L., Rubin K., Larsson E., Wigzell H. T lymphocytes in collagen II-induced arthritis in mice. Characterization of arthritogenic collagen II-specific T-cell lines and clones. Scand J Immunol. 1985 Sep;22(3):295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- Horne C. H., Thomson A. W., Hunter C. B., Tunstall A. M., Towler C. M., Billingham M. E. Pregnancy-associated alpha 2-glycoprotein (alpha 2-PAG) and various acute phase reactants in rheumatoid arthritis and osteoarthritis. Biomedicine. 1979 Jun;30(2):90–94. [PubMed] [Google Scholar]
- Lee S. H., Matsuyama T., Logalbo P., Silver J., Winchester R. J. Ia antigens and susceptibility to rheumatoid arthritis. Clin Rheum Dis. 1985 Dec;11(3):645–664. [PubMed] [Google Scholar]
- Mor-Yosef S., Navot D., Rabinowitz R., Schenker J. G. Collagen diseases in pregnancy. Obstet Gynecol Surv. 1984 Feb;39(2):67–84. doi: 10.1097/00006254-198402000-00002. [DOI] [PubMed] [Google Scholar]
- Mortimer G., Stimson W. H., Hunter I. C., Govan A. D. A role for amniotic epithelium in control of human parturition. Lancet. 1985 May 11;1(8437):1074–1075. doi: 10.1016/s0140-6736(85)92372-4. [DOI] [PubMed] [Google Scholar]
- Ostensen M., Husby G. A prospective clinical study of the effect of pregnancy on rheumatoid arthritis and ankylosing spondylitis. Arthritis Rheum. 1983 Sep;26(9):1155–1159. doi: 10.1002/art.1780260915. [DOI] [PubMed] [Google Scholar]
- Ostensen M., Husby G. Pregnancy and rheumatic disease. A review of recent studies in rheumatoid arthritis and ankylosing spondylitis. Klin Wochenschr. 1984 Oct 1;62(19):891–895. doi: 10.1007/BF01727437. [DOI] [PubMed] [Google Scholar]
- Ostensen M., von Schoultz B., Husby G. Comparison between serum alpha 2-pregnancy-associated globulin and activity of rheumatoid arthritis and ankylosing spondylitis during pregnancy. Scand J Rheumatol. 1983;12(3):315–318. doi: 10.3109/03009748309098556. [DOI] [PubMed] [Google Scholar]
- Persellin R. H., Rhodes J. Inhibition of human monocyte Fc receptor and HLA-DR antigen expression by pregnancy alpha-2 glycoprotein. Clin Exp Immunol. 1981 Nov;46(2):350–354. [PMC free article] [PubMed] [Google Scholar]
- Persellin R. H. The effect of pregnancy on rheumatoid arthritis. Bull Rheum Dis. 1976;27(9):922–927. [PubMed] [Google Scholar]
- Phadke K., Carlson D. G., Gitter B. D., Butler L. D. Role of interleukin 1 and interleukin 2 in rat and mouse arthritis models. J Immunol. 1986 Jun 1;136(11):4085–4091. [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley M. G., Panayi G. S., Nicholas N. S., Murphy J. Mechanisms of macrophage activation in rheumatoid arthritis: the role of gamma-interferon. Clin Exp Immunol. 1986 Mar;63(3):587–593. [PMC free article] [PubMed] [Google Scholar]
- Stuart J. M., Dixon F. J. Serum transfer of collagen-induced arthritis in mice. J Exp Med. 1983 Aug 1;158(2):378–392. doi: 10.1084/jem.158.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. W., Horne C. H. Biological and clinical significance of pregnancy-associated alpha2-glycorprotein (alpha/-PAG)-a review. Invest Cell Pathol. 1980 Jul-Sep;3(3):295–309. [PubMed] [Google Scholar]
- Trentham D. E. Collagen arthritis as a relevant model for rheumatoid arthritis. Arthritis Rheum. 1982 Aug;25(8):911–916. doi: 10.1002/art.1780250801. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger A., Kay A., Griffin A. J., Panayi G. S. Disease activity and pregnancy associated alpha 2-glycoprotein in rheumatoid arthritis during pregnancy. Br Med J (Clin Res Ed) 1983 Mar 5;286(6367):750–752. doi: 10.1136/bmj.286.6367.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner B. P., Mattaliano R. J., Hession C., Cate R. L., Tizard R., Sinclair L. K., Foeller C., Chow E. P., Browing J. L., Ramachandran K. L. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]
- Wooley P. H., Luthra H. S., Lafuse W. P., Huse A., Stuart J. M., David C. S. Type II collagen-induced arthritis in mice. III. Suppression of arthritis by using monoclonal and polyclonal anti-Ia antisera. J Immunol. 1985 Apr;134(4):2366–2374. [PubMed] [Google Scholar]
- Wooley P. H., Luthra H. S., Stuart J. M., David C. S. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J Exp Med. 1981 Sep 1;154(3):688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]