Abstract
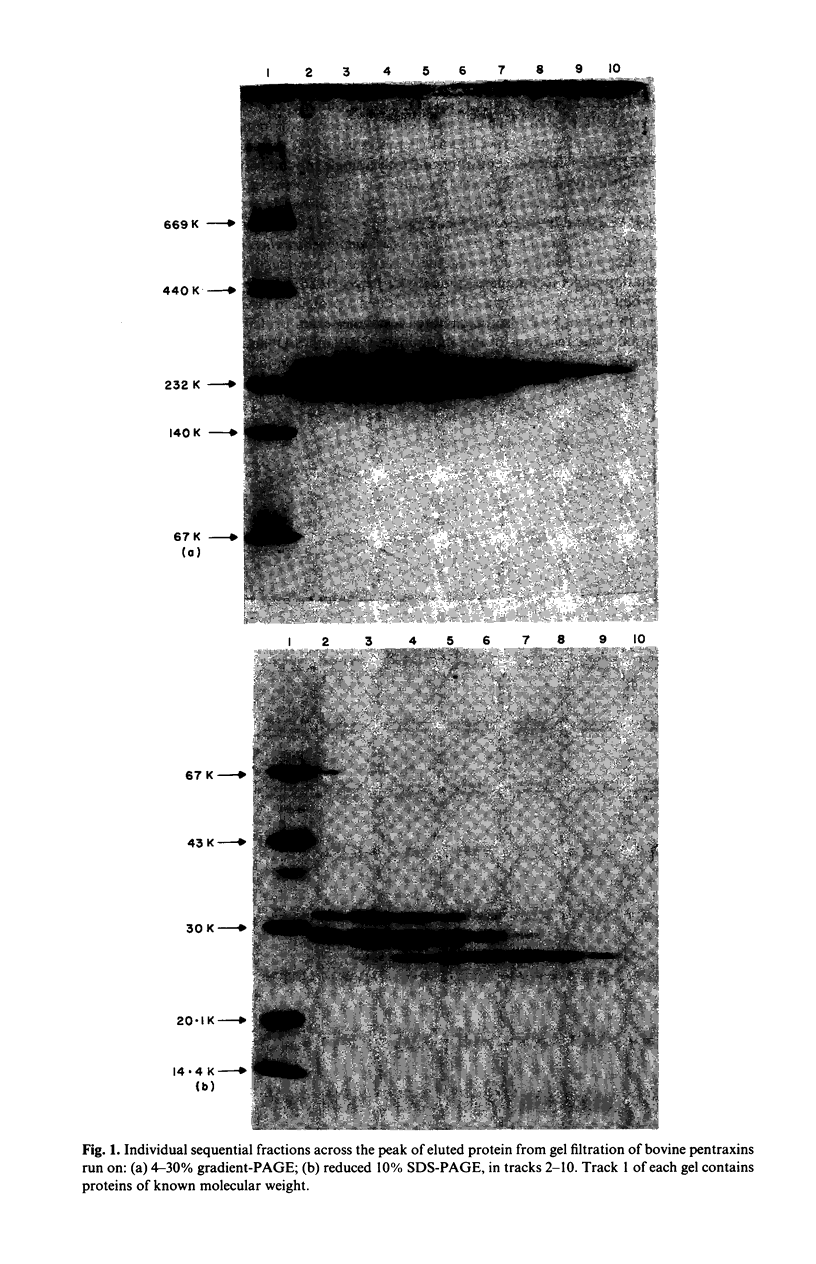
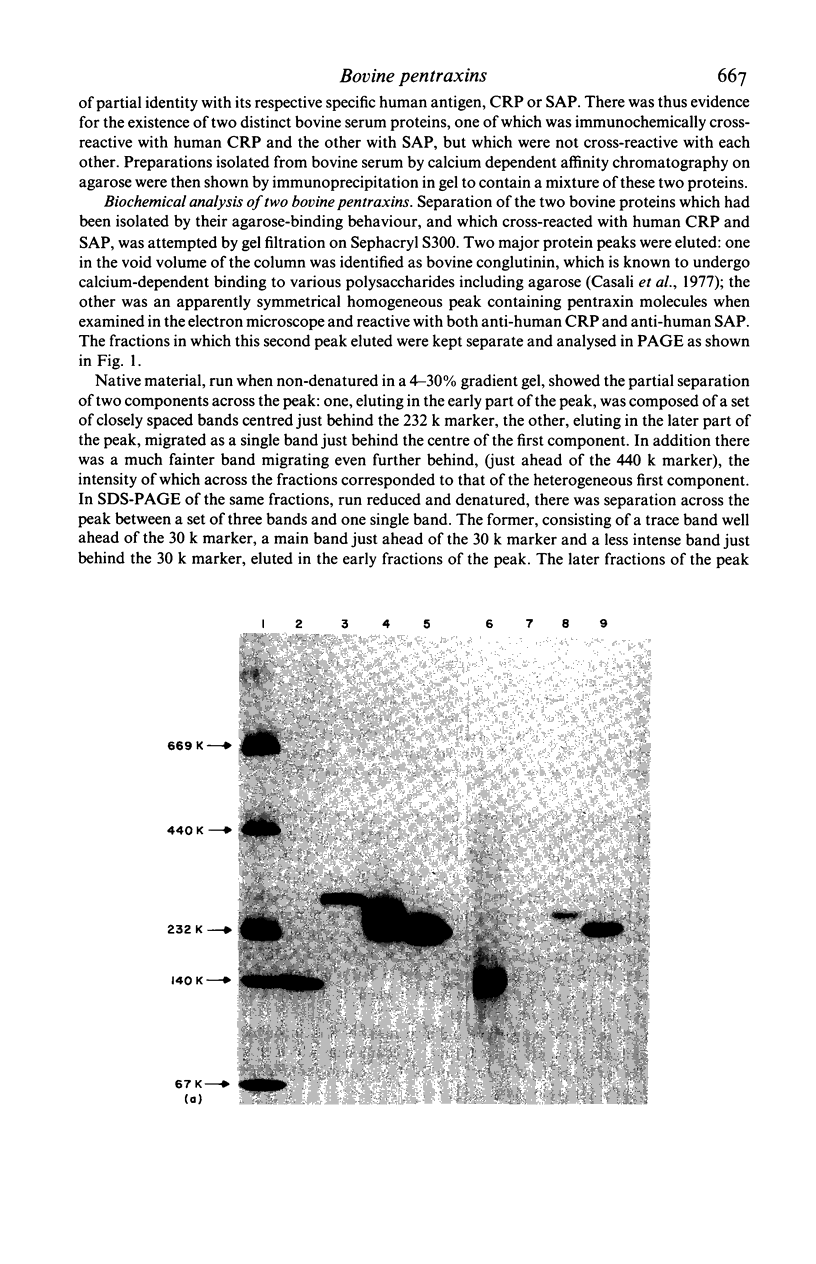
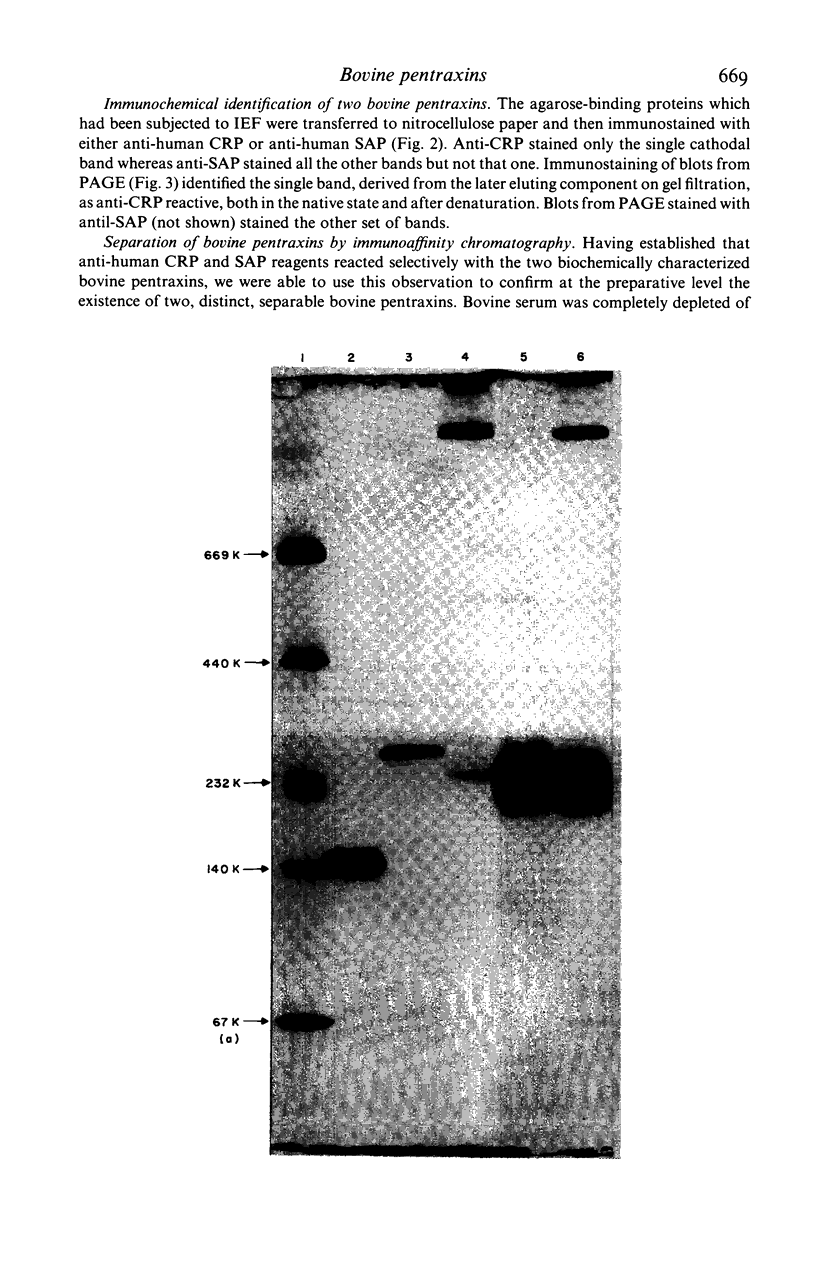
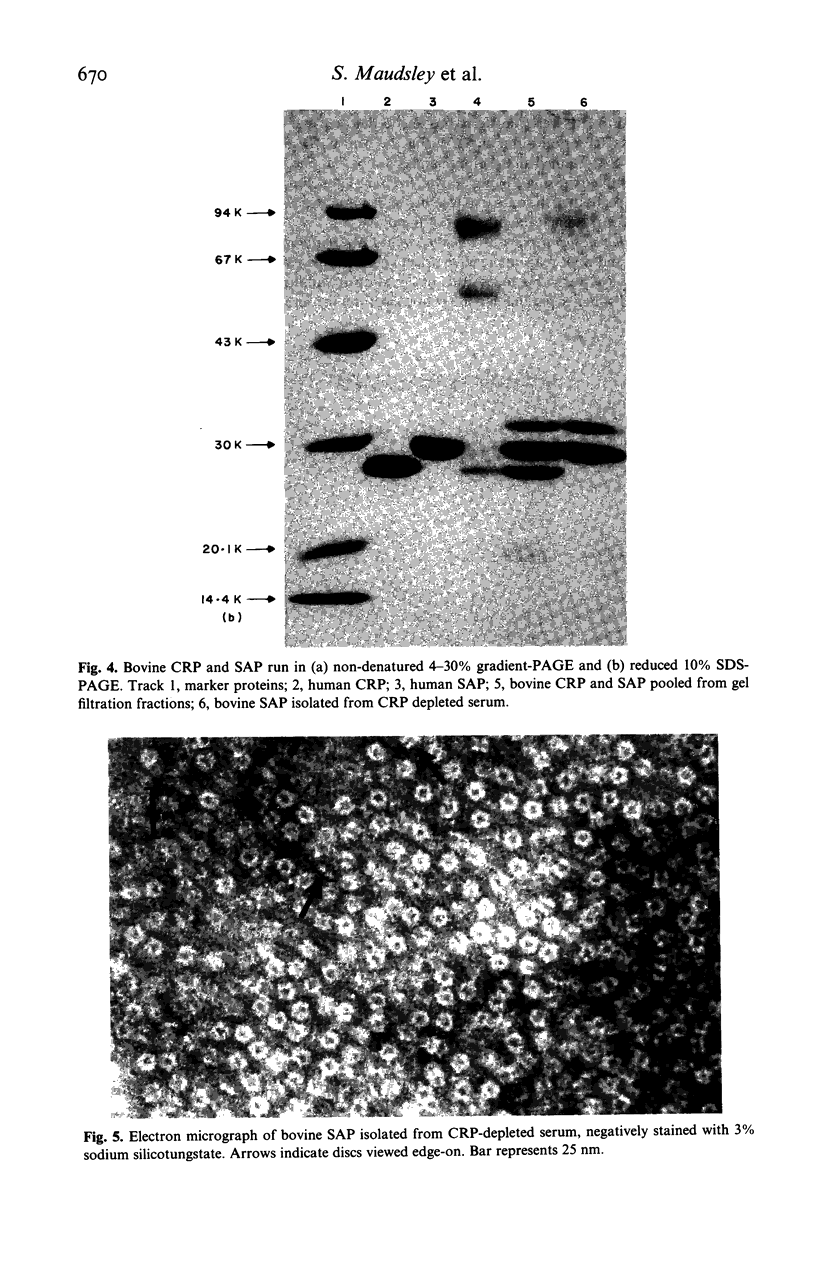
Two distinct pentraxin proteins were isolated from bovine serum by calcium-dependent affinity chromatography on high pyruvate agarose gel. One of these proteins cross-reacted specifically with certain rabbit anti-human CRP antisera and was therefore designated as bovine CRP. The other cross-reacted specifically with a sheep anti-human SAP antiserum and was therefore designated as bovine SAP. Although the mixture of these two pentraxins was not resolved by gel filtration chromatography, they were separated by solid phase absorption of the CRP with immobilized rabbit anti-human CRP antibodies. Their identity as pentraxins was confirmed by their electron microscopic appearance. Bovine CRP was composed of a single type of non-glycosylated subunit whilst bovine SAP contained two major types of glycosylated subunits and a minor polypeptide, the glycosylation of which was not determined. Serum concentrations were in the range of 5-40 mg/l and neither protein behaved as an acute phase reactant. No bovine serum protein undergoing calcium-dependent binding to phosphoryl choline or pneumococcal C-polysaccharide was obtained.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz M. L., de Beer F. C., Feinstein A., Munn E. A., Milstein C. P., Fletcher T. C., March J. F., Taylor J., Bruton C., Clamp J. R. Phylogenetic aspects of C-reactive protein and related proteins. Ann N Y Acad Sci. 1982;389:49–75. doi: 10.1111/j.1749-6632.1982.tb22125.x. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Breathnach S. M., Melrose S. M., Bhogal B., de Beer F. C., Dyck R. F., Tennent G., Black M. M., Pepys M. B. Amyloid P component is located on elastic fibre microfibrils in normal human tissue. Nature. 1981 Oct 22;293(5834):652–654. doi: 10.1038/293652a0. [DOI] [PubMed] [Google Scholar]
- Casali P., Bossus A., Carpentier N. A., Lambert P. H. Solid-phase enzyme immunoassay or radioimmunoassay for the detection of immune complexes based on their recognition by conglutinin: conglutinin-binding test. A comparative study with 125I-labelled Clq binding and Raji-cell RIA tests. Clin Exp Immunol. 1977 Aug;29(2):342–354. [PMC free article] [PubMed] [Google Scholar]
- Caspi D., Baltz M. L., Snel F., Gruys E., Niv D., Batt R. M., Munn E. A., Buttress N., Pepys M. B. Isolation and characterization of C-reactive protein from the dog. Immunology. 1984 Oct;53(2):307–313. [PMC free article] [PubMed] [Google Scholar]
- De Beer F. C., Pepys M. B. Isolation of human C-reactive protein and serum amyloid P component. J Immunol Methods. 1982;50(1):17–31. doi: 10.1016/0022-1759(82)90300-3. [DOI] [PubMed] [Google Scholar]
- Dowton S. B., Woods D. E., Mantzouranis E. C., Colten H. R. Syrian hamster female protein: analysis of female protein primary structure and gene expression. Science. 1985 Jun 7;228(4704):1206–1208. doi: 10.1126/science.2408337. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Neville D. M., Jr Glycoproteins of cell surfaces. A comparative study of three different cell surfaces of the rat. J Biol Chem. 1971 Oct 25;246(20):6339–6346. [PubMed] [Google Scholar]
- Hind C. R., Collins P. M., Renn D., Cook R. B., Caspi D., Baltz M. L., Pepys M. B. Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. J Exp Med. 1984 Apr 1;159(4):1058–1069. doi: 10.1084/jem.159.4.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANIGAN D. T. EXPERIMENTAL AMYLOIDOSIS: STUDIES WITH A MODIFIED CASEIN METHOD, CASEIN HYDROLYSATE AND GELATIN. Am J Pathol. 1965 Jul;47:159–171. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mantzouranis E. C., Dowton S. B., Whitehead A. S., Edge M. D., Bruns G. A., Colten H. R. Human serum amyloid P component. cDNA isolation, complete sequence of pre-serum amyloid P component, and localization of the gene to chromosome 1. J Biol Chem. 1985 Jun 25;260(12):7752–7756. [PubMed] [Google Scholar]
- Maudsley S., Hind C. R., Munn E. A., Buttress N., Pepys M. B. Isolation and characterization of guinea-pig serum amyloid P component. Immunology. 1986 Oct;59(2):317–322. [PMC free article] [PubMed] [Google Scholar]
- Osmand A. P., Friedenson B., Gewurz H., Painter R. H., Hofmann T., Shelton E. Characterization of C-reactive protein and the complement subcomponent C1t as homologous proteins displaying cyclic pentameric symmetry (pentraxins). Proc Natl Acad Sci U S A. 1977 Feb;74(2):739–743. doi: 10.1073/pnas.74.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C., Ashley M. J. Isolation of C-reactive protein by affinity chromatography. Clin Exp Immunol. 1977 Oct;30(1):32–37. [PMC free article] [PubMed] [Google Scholar]
- Pepys M. B., Dash A. C., Fletcher T. C., Richardson N., Munn E. A., Feinstein A. Analogues in other mammals and in fish of human plasma proteins, C-reactive protein and amyloid P component. Nature. 1978 May 11;273(5658):168–170. doi: 10.1038/273168a0. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., De Beer F. C., Milstein C. P., March J. F., Feinstein A., Butress N., Clamp J. R., Taylor J., Bruton C., Fletcher T. C. C-reactive protein and serum amyloid P component in the plaice (Pleuronectes platessa L.), a marine teleost, are homologous with their human counterparts. Biochim Biophys Acta. 1982 May 21;704(1):123–133. doi: 10.1016/0167-4838(82)90139-x. [DOI] [PubMed] [Google Scholar]
- Robey F. A., Jones K. D., Tanaka T., Liu T. Y. Binding of C-reactive protein to chromatin and nucleosome core particles. A possible physiological role of C-reactive protein. J Biol Chem. 1984 Jun 10;259(11):7311–7316. [PubMed] [Google Scholar]
- Robey F. A., Liu T. Y. Limulin: a C-reactive protein from Limulus polyphemus. J Biol Chem. 1981 Jan 25;256(2):969–975. [PubMed] [Google Scholar]
- Robey F. A., Tanaka T., Liu T. Y. Isolation and characterization of two major serum proteins from the dogfish, Mustelus canis, C-reactive protein and amyloid P component. J Biol Chem. 1983 Mar 25;258(6):3889–3894. [PubMed] [Google Scholar]
- Volanakis J. E., Kaplan M. H. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971 Feb;136(2):612–614. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- de Beer F. C., Baltz M. L., Holford S., Feinstein A., Pepys M. B. Fibronectin and C4-binding protein are selectively bound by aggregated amyloid P component. J Exp Med. 1981 Oct 1;154(4):1134–1139. doi: 10.1084/jem.154.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer F. C., Baltz M. L., Munn E. A., Feinstein A., Taylor J., Bruton C., Clamp J. R., Pepys M. B. Isolation and characterization of C-reactive protein and serum amyloid P component in the rat. Immunology. 1982 Jan;45(1):55–70. [PMC free article] [PubMed] [Google Scholar]