Abstract
Nasal biopsy specimens from 15 adult patients with selective IgA deficiency but normal IgG-subclass levels were examined by immunohistochemistry for the presence of immunocytes producing various Ig isotypes. The mucosal samples were completely IgA-deficient except in two cases where 0.9% and 8.4% IgA cells were found, respectively (normal, 69.8%). Numerous IgG- (mainly IgG1-) producing cells were present in 10 samples; in five of these there were additional IgM- but virtually no IgD-producing cells, whereas in the other five a marked dominance of the IgD over the IgM isotype was seen. The latter category of patients had more upper airways infections (recurrent acute rhinosinusitis, otitis media, and tonsillitis) than the former, who had no recurrent upper respiratory tract infections except one patient with recurrent acute rhinosinusitis. The five remaining samples, which contained very few Ig-producing cells, were derived from patients with even more frequent infections than those showing IgD predominance. Our results indicate that IgM acts as a compensatory secretory Ig in the upper respiratory tract of some IgA-deficient subjects. However, immunoregulatory events favouring local IgD responses apparently do not support mucosal defence satisfactorily, either because local production of IgM is hampered or because IgD (which is not a secretory Ig) blocks complement-dependent reactions mediated by IgG and IgM antibodies within the mucosa.
Full text
PDF






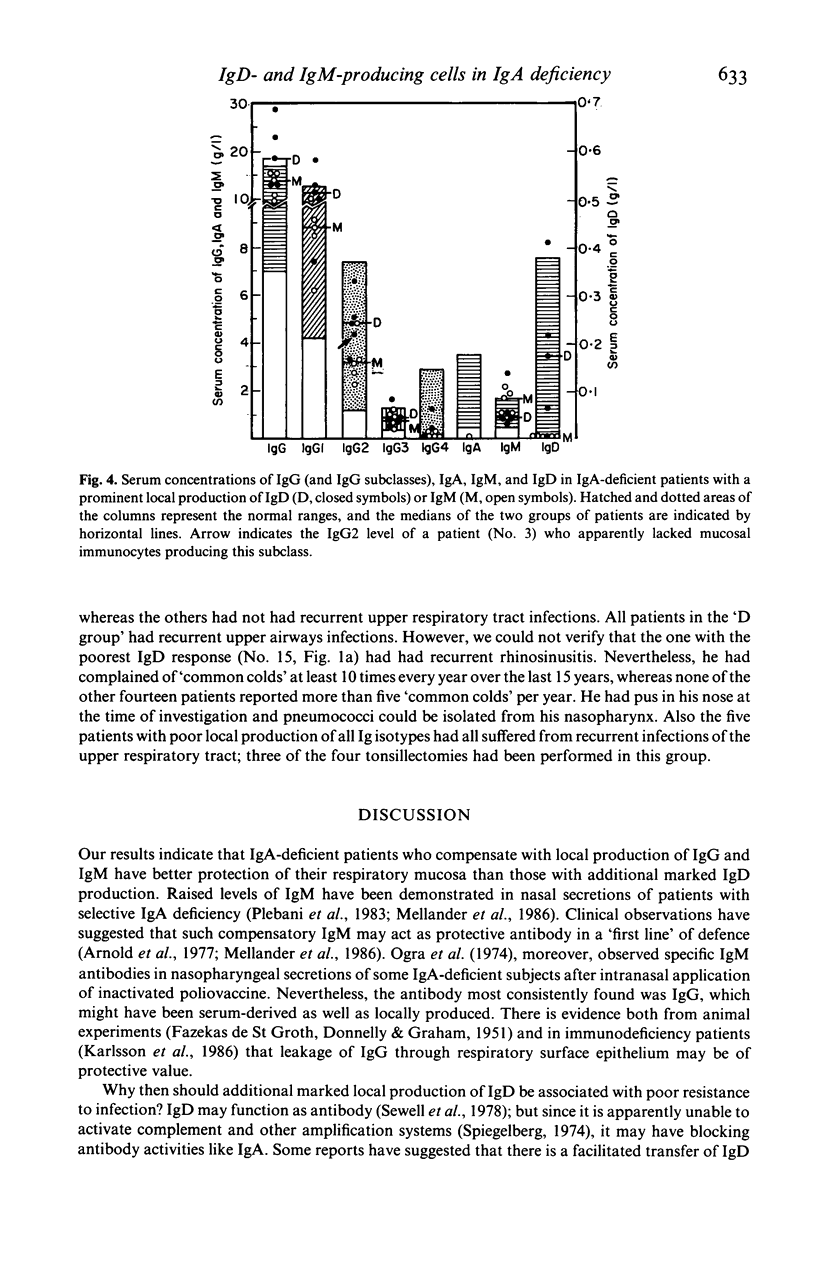



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold R. R., Cole M. F., Prince S., McGhee J. R. Secretory IgM antibodies to Streptococcus mutans in subjects with selective IgA deficiency. Clin Immunol Immunopathol. 1977 Nov;8(3):475–486. doi: 10.1016/0090-1229(77)90011-3. [DOI] [PubMed] [Google Scholar]
- Bjerke K., Brandtzaeg P. Immunoglobulin- and J chain-producing cells associated with lymphoid follicles in the human appendix, colon and ileum, including Peyer's patches. Clin Exp Immunol. 1986 May;64(2):432–441. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P., Gjeruldsen S. T., Korsrud F., Baklien K., Berdal P., Ek J. The human secretory immune system shows striking heterogeneity with regard to involvement of J chain-positive IgD immunocytes. J Immunol. 1979 Feb;122(2):503–510. [PubMed] [Google Scholar]
- Brandtzaeg P. Human secretory component--VI. Immunoglobulin-binding properities. Immunochemistry. 1977 Mar;14(3):179–188. doi: 10.1016/0019-2791(77)90192-6. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Immunohistochemical characterization of intracellular J-chain and binding site for secretory component (SC) in human immunoglobulin (Ig)-producing cells. Mol Immunol. 1983 Sep;20(9):941–966. doi: 10.1016/0161-5890(83)90036-6. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Kett K., Rognum T. O., Söderström R., Björkander J., Söderström T., Petrusson B., Hanson L. A. Distribution of mucosal IgA and IgG subclass-producing immunocytes and alterations in various disorders. Monogr Allergy. 1986;20:179–194. [PubMed] [Google Scholar]
- Brandtzaeg P., Korsrud F. R. Significance of different J chain profiles in human tissues: generation of IgA and IgM with binding site for secretory component is related to the J chain expressing capacity of the total local immunocyte population, including IgG and IgD producing cells, and depends on the clinical state of the tissue. Clin Exp Immunol. 1984 Dec;58(3):709–718. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Prolonged incubation time in immunohistochemistry: effects on fluorescence staining of immunoglobulins and epithelial components in ethanol- and formaldehyde-fixed paraffin-embedded tissues. J Histochem Cytochem. 1981 Nov;29(11):1302–1315. doi: 10.1177/29.11.7033362. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Rognum T. O. Evaluation of tissue preparation methods and paired immunofluorescence staining for immunocytochemistry of lymphomas. Histochem J. 1983 Jul;15(7):655–689. doi: 10.1007/BF01002987. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P. Role of J chain and secretory component in receptor-mediated glandular and hepatic transport of immunoglobulins in man. Scand J Immunol. 1985 Aug;22(2):111–146. doi: 10.1111/j.1365-3083.1985.tb01866.x. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Surjan L., Jr, Berdal P. Immunoglobulin systems of human tonsils. I. Control subjects of various ages: quantification of Ig-producing cells, tonsillar morphometry and serum Ig concentrations. Clin Exp Immunol. 1978 Mar;31(3):367–381. [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. The secretory immune system of lactating human mammary glands compared with other exocrine organs. Ann N Y Acad Sci. 1983 Jun 30;409:353–382. doi: 10.1111/j.1749-6632.1983.tb26883.x. [DOI] [PubMed] [Google Scholar]
- FAZEKAS DE ST GROTH S., DONNELLEY M., GRAHAM D. M. Studies in experimental immunology of influenza. VIII. Pathotopic adjuvants. Aust J Exp Biol Med Sci. 1951 Sep;29(5):323–327. doi: 10.1038/icb.1951.39. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Rabbitts T. H. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing gamma, epsilon and alpha genes. Nature. 1982 Dec 23;300(5894):709–713. doi: 10.1038/300709a0. [DOI] [PubMed] [Google Scholar]
- Heddle R. J., Kwitko A. O., Shearman D. J. Specific IgM and IgG antibodies in IgA deficiency. Clin Exp Immunol. 1980 Sep;41(3):453–458. [PMC free article] [PubMed] [Google Scholar]
- Karlsson G., Petruson B., Björkander J., Hanson L. A. Infections of the nose and paranasal sinuses in adult patients with immunodeficiency. Arch Otolaryngol. 1985 May;111(5):290–293. doi: 10.1001/archotol.1985.00800070042003. [DOI] [PubMed] [Google Scholar]
- Keller M. A., Heiner D. C., Myers A. S., Reisinger D. M. IgD in human colostrum. Pediatr Res. 1985 Jan;19(1):122–126. doi: 10.1203/00006450-198501000-00032. [DOI] [PubMed] [Google Scholar]
- Kett K., Brandtzaeg P., Radl J., Haaijman J. J. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J Immunol. 1986 May 15;136(10):3631–3635. [PubMed] [Google Scholar]
- Korsrud F. R., Brandtzaeg P. Immune systems of human nasopharyngeal and palatine tonsils: histomorphometry of lymphoid components and quantification of immunoglobulin-producing cells in health and disease. Clin Exp Immunol. 1980 Feb;39(2):361–370. [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mellander L., Björkander J., Carlsson B., Hanson L. A. Secretory antibodies in IgA-deficient and immunosuppressed individuals. J Clin Immunol. 1986 Jul;6(4):284–291. doi: 10.1007/BF00917328. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Coppola P. R., MacGillivray M. H., Dzierba J. L. Mechanism of mucosal immunity to viral infections in gammaA immunoglobulin-deficiency syndromes. Proc Soc Exp Biol Med. 1974 Mar;145(3):811–816. doi: 10.3181/00379727-145-37900. [DOI] [PubMed] [Google Scholar]
- Ogra P. L. Effect of tonsillectomy and adenoidectomy on nasopharyngeal antibody response to poliovirus. N Engl J Med. 1971 Jan 14;284(2):59–64. doi: 10.1056/NEJM197101142840201. [DOI] [PubMed] [Google Scholar]
- Oxelius V. A. Crossed immunoelectrophoresis and electroimmunoassay of human IgG subclasses. Acta Pathol Microbiol Scand C. 1978 Jun;86C(3):109–116. doi: 10.1111/j.1699-0463.1978.tb02567.x. [DOI] [PubMed] [Google Scholar]
- Plebani A., Mira E., Mevio E., Monafo V., Notarangelo L. D., Avanzini A., Ugazio A. G. IgM and IgD concentrations in the serum and secretions of children with selective IgA deficiency. Clin Exp Immunol. 1983 Sep;53(3):689–696. [PMC free article] [PubMed] [Google Scholar]
- Sewell H. F., Chambers L., Maxwell V., Matthews J. B., Jefferis R. The natural antibody response to E. coli includes antibodies of the IgD class. Clin Exp Immunol. 1978 Jan;31(1):104–110. [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L. Biological activities of immunoglobulins of different classes and subclasses. Adv Immunol. 1974;19(0):259–294. doi: 10.1016/s0065-2776(08)60254-0. [DOI] [PubMed] [Google Scholar]
- Steele M. G., Leslie G. A. Immunoglobulin D in rat serum, saliva and milk. Immunology. 1985 Aug;55(4):571–577. [PMC free article] [PubMed] [Google Scholar]
- Wakelin D., Selby G. R. The induction of immunological tolerance to the parasitic nematode Trichuris muris in cortisone-treated mice. Immunology. 1974 Jan;26(1):1–10. [PMC free article] [PubMed] [Google Scholar]



