Abstract
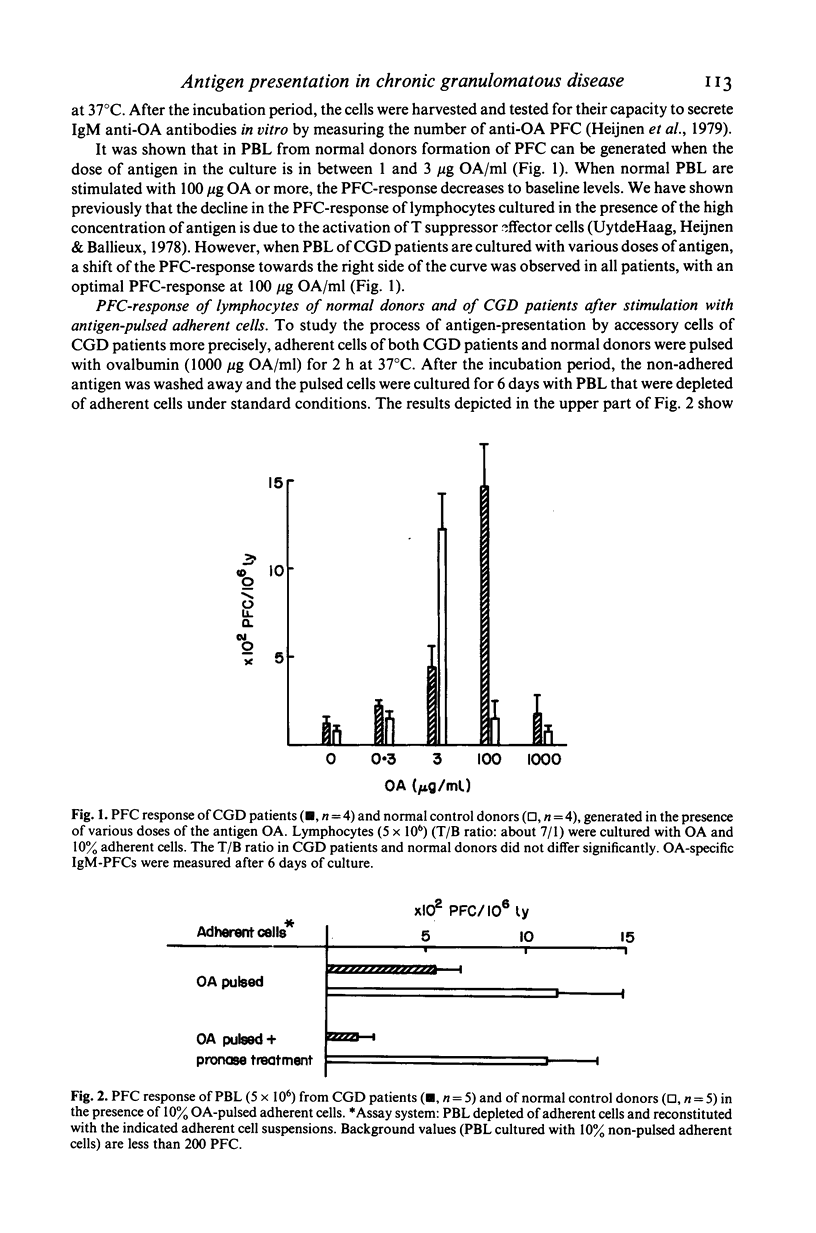
Phagocytic cells of patients with chronic granulomatous disease (CGD) are severely impaired in the killing process, on the basis of defective oxygen metabolism. In this study we investigated the antigen-presenting function of monocytes (i.e. adherent cells) of CGD patients. Adherent cells of CGD patients were investigated for their capacity to present ovalbumin (OA) in such a way that T helper cells are activated and OA-specific IgM-plaque forming cells (PFC) are generated. The results showed that the dose of OA required for optimal PFC responses was 20-25 times higher than the dose of antigen which is necessary for induction of PFCs from peripheral blood mononuclear cells of normal donors. We could show that the CGD monocytes were responsible for the observed shift which indicates peculiar antigen-presenting capacities. The finding could be mimicked in normal donors by treating adherent cells with phenylbutazone, a drug known to interfere with oxygen-dependent degradation of micro-organisms. Moreover, our results suggested that CGD monocytes and phenylbutazone-treated monocytes of normal donors absorb OA but do not re-express the processed antigen on the membrane. We conclude that our system used to study antigen presentation allowed the discovery of altered antigen presentation of CGD monocytes most probably on the basis of defective antigen processing. However, antigen presentation is not entirely absent, since relatively high doses of OA induced normal PFC responses in CGD mononuclear cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Ballieux R. E., Heijnen C. J. Immunoregulatory T cell subpopulations in man: dissection by monoclonal antibodies and Fc-receptors. Immunol Rev. 1983;74:5–28. doi: 10.1111/j.1600-065x.1983.tb01082.x. [DOI] [PubMed] [Google Scholar]
- Ballieux R. E., Heijnen C. J., Uytdehaag F., Zegers B. J. Regulation of B cell activity in man: role of T cells. Immunol Rev. 1979;45:3–39. doi: 10.1111/j.1600-065x.1979.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Goding J. W. The chromic chloride method of coupling antigens to erythrocytes: definition of some important parameters. J Immunol Methods. 1976;10(1):61–66. doi: 10.1016/0022-1759(76)90007-7. [DOI] [PubMed] [Google Scholar]
- Hart L. A., Heijnen C. J., Zijlstra J., Ballieux R. E. Analysis of the antigen-induced in vitro differentiation of human peripheral blood B cells: stimulation with ovalbumin induces the transition from the resting state into the excited state. Clin Exp Immunol. 1985 Sep;61(3):648–656. [PMC free article] [PubMed] [Google Scholar]
- Heijnen C. J., UytdeHaag F., Gmelig-Meyling F. H., Ballieux R. E. Localization of human antigen-specific helper and suppressor function in distinct T-cell subpopulations. Cell Immunol. 1979 Mar 15;43(2):282–292. doi: 10.1016/0008-8749(79)90173-4. [DOI] [PubMed] [Google Scholar]
- Heijnen C. J., Uytdehaag F., Pot K. H., Ballieux R. E. Antigen-specific human T cell factors. I. T cell helper factor: biololgic properties. J Immunol. 1981 Feb;126(2):497–502. [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg C. O. Influence of therapeutic concentrations of phenylbutazone on granulocyte function. Acta Pathol Microbiol Scand B. 1975 Apr;83(2):100–102. doi: 10.1111/j.1699-0463.1975.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Lu C. Y., Allen P. M. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984 Jan;132(1):1–5. [PubMed] [Google Scholar]
- UytdeHaag F., Heynen C. J., Ballieux R. E. Induction of antigen-specific human suppressor T lymphocytes in vitro. Nature. 1978 Feb 9;271(5645):556–557. doi: 10.1038/271556a0. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]
- van Tol M. J., Zijlstra J., Thomas C. M., Zegers B. J., Ballieux R. E. Distinct role of neonatal and adult monocytes in the regulation of the in vitro antigen-induced plaque-forming cell response in man. J Immunol. 1984 Oct;133(4):1902–1908. [PubMed] [Google Scholar]


