Abstract
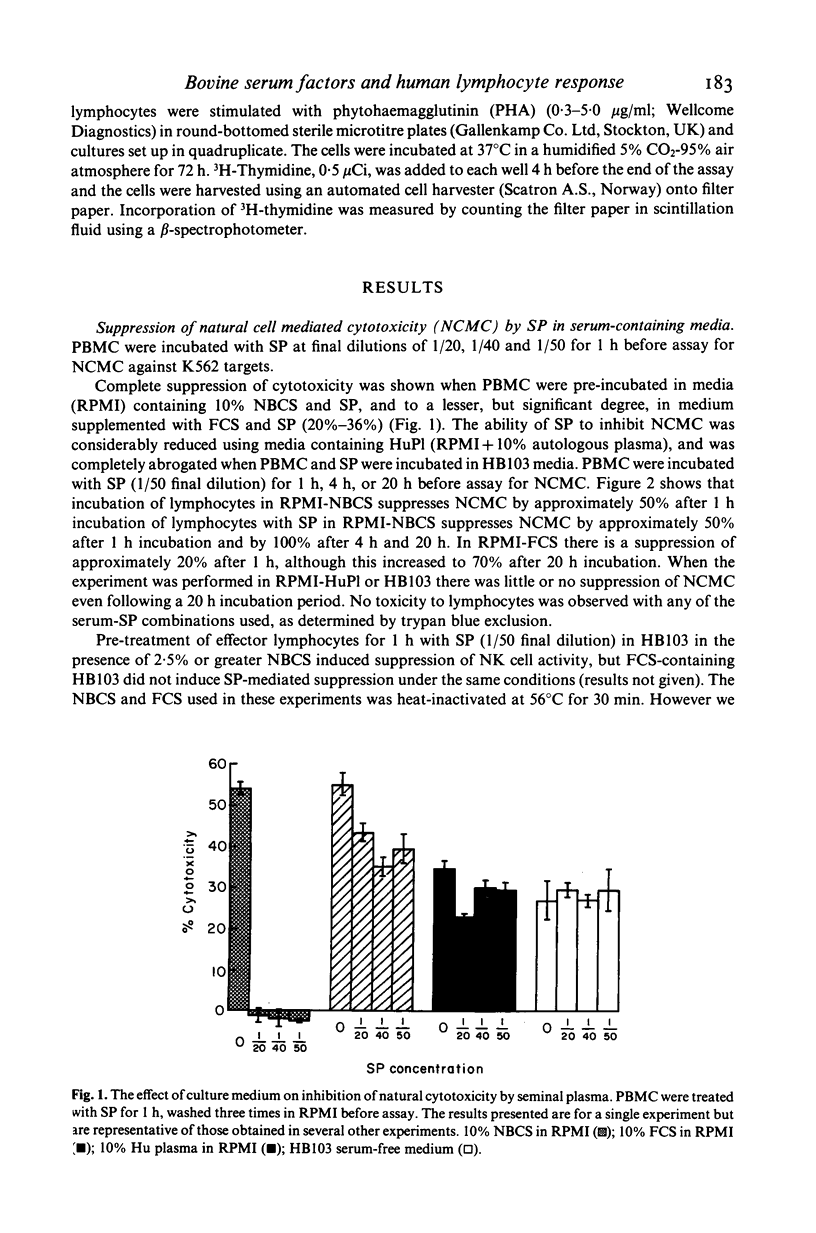
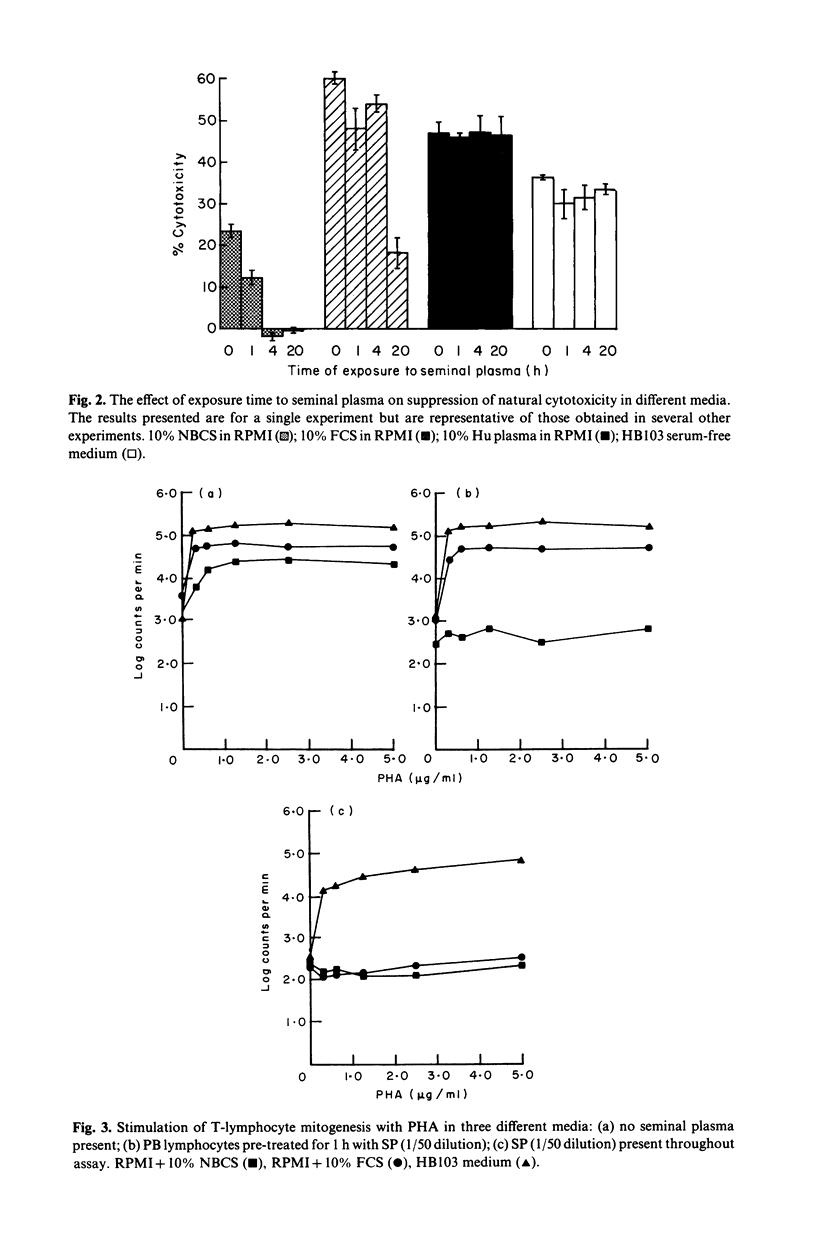
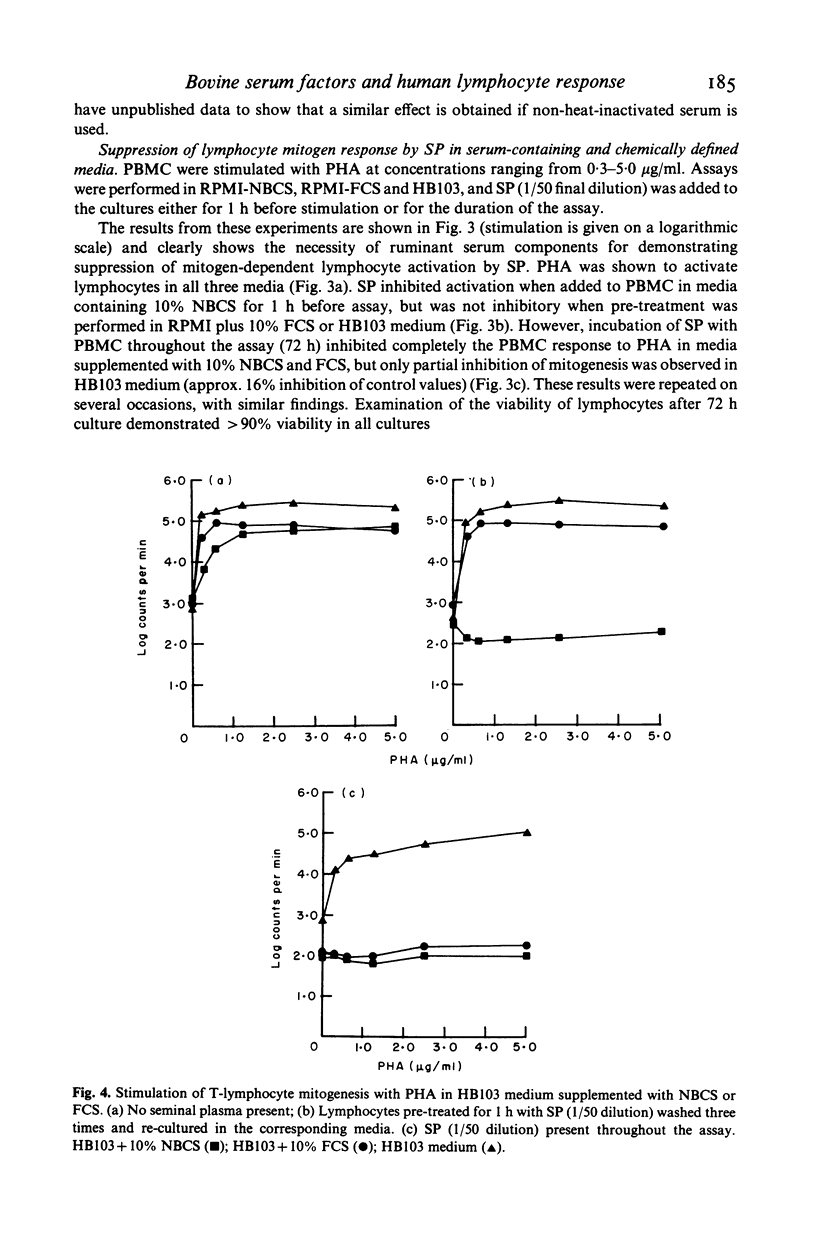
The effect of bovine sera on the ability of human seminal plasma (SP) to suppress lymphocyte responses was investigated. Marked suppression of natural cell-mediated cytotoxicity (NCMC) against K562 targets was observed when effectors were pretreated for 1 h with SP (1:50 dilution) in the presence of 10% newborn calf serum (NBCS). Some suppression of natural cytotoxicity was observed when the effectors were treated with SP in the presence of 10% fetal calf serum (FCS) and this suppression was greater if the length of pretreatment with SP was increased to 20 h. Suppression of NCMC did not occur, or was considerably less, when the effectors were treated with SP in the presence of 10% autologous human plasma or in HB103 serum-free medium. The effect of bovine sera on suppression of T lymphocyte response to mitogen was also examined. Pre-treatment of lymphocytes with SP (1:50 dilution) for 1 h in the presence of 10% NBCS was sufficient to abrogate completely the stimulatory effect of PHA. In the presence of 10% FCS it was necessary for SP to be present throughout the assay for suppression to occur. In HB103 medium, stimulation was only slightly decreased below control values when SP was present throughout the assay, but suppression was considerably less than that obtained upon addition of NBCS or FCS to the culture medium. These findings imply that suppression of lymphocyte activity by SP is dependent on the presence of exogenous serum co-factors and in the light of this finding, the clinical relevance of SP suppression may require re-examination.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Tarter T. H. Immunosuppressive effects of mouse seminal plasma components in vivo and in vitro. J Immunol. 1982 Feb;128(2):535–539. [PubMed] [Google Scholar]
- Byrd W. J., Jacobs D. M., Amoss M. S. Synthetic polyamines added to cultures containing bovine sera reversibly inhibit in vitro parameters of immunity. Nature. 1977 Jun 16;267(5612):621–623. doi: 10.1038/267621a0. [DOI] [PubMed] [Google Scholar]
- Chvapil M., Stankova L., Bernhard D. S., Zukoski C. F., Drach G. W. Effect of prostatic fluid and its fraction on some functions of peritoneal macrophages. Invest Urol. 1977 Sep;15(2):173–179. [PubMed] [Google Scholar]
- Franken D. R., Slabber C. F. Reproductive immunology: 1. The effect of different protein concentrations of seminal plasma on in vitro lymphocyte cultures. Andrologia. 1981 Sep-Oct;13(5):504–507. doi: 10.1111/j.1439-0272.1981.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Franken D. R., Slabber C. F. Reproductive immunology: 3. the effect of seminal plasma from oligozoospermic men on in vitro lymphocyte cultures. Andrologia. 1982 Jan-Feb;14(1):8–11. doi: 10.1111/j.1439-0272.1982.tb03087.x. [DOI] [PubMed] [Google Scholar]
- Hölttä E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977 Jan 11;16(1):91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- Illei G., Morgan D. M. Polyamine oxidase activity in human pregnancy serum. Br J Obstet Gynaecol. 1979 Nov;86(11):878–881. doi: 10.1111/j.1471-0528.1979.tb10715.x. [DOI] [PubMed] [Google Scholar]
- James K., Szymaniec S. Human seminal plasma is a potent inhibitor of natural killer cell activity in vitro. J Reprod Immunol. 1985 Aug;8(1):61–70. doi: 10.1016/0165-0378(85)90078-6. [DOI] [PubMed] [Google Scholar]
- Labib R. S., Tomasi T. B., Jr Enzymatic oxidation of polyamines. Relationship to immunosuppressive properties. Eur J Immunol. 1981 Mar;11(3):266–269. doi: 10.1002/eji.1830110318. [DOI] [PubMed] [Google Scholar]
- Lord E. M., Sensabaugh G. F., Stites D. P. Immunosuppressive activity of human seminal plasma. I. Inhibition of in vitro lymphocyte activation. J Immunol. 1977 May;118(5):1704–1711. [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Majumdar S., Bapna B. C., Mapa M. K., Gupta A. N., Devi P. K., Subrahmanyam D. Effect of seminal plasma and its fractions on in vitro blastogenic response to mitogen. Int J Fertil. 1982;27(4):224–228. [PubMed] [Google Scholar]
- Marcus Z. H., Freisheim J. H., Houk J. L., Herman J. H., Hess E. V. In vitro studies in reproductive immunology. 1. Suppression of cell-mediated immune response by human spermatozoa and fractions isolated from human seminal plasma. Clin Immunol Immunopathol. 1978 Mar;9(3):318–326. doi: 10.1016/0090-1229(78)90103-4. [DOI] [PubMed] [Google Scholar]
- Petersen B. H., Lammel C. J., Stites D. P., Brooks G. F. Human seminal plasma inhibition of complement. J Lab Clin Med. 1980 Oct;96(4):582–591. [PubMed] [Google Scholar]
- Pitout M. J., Jordaan J. H. Partial purification of an antimitogenic factor from human semen. Int J Biochem. 1976;7(3-4):149–151. doi: 10.1016/0020-711x(76)90012-4. [DOI] [PubMed] [Google Scholar]
- Rees R. C., Vallely P., Clegg A., Potter C. W. Suppression of natural and activated human antitumour cytotoxicity by human seminal plasma. Clin Exp Immunol. 1986 Mar;63(3):687–695. [PMC free article] [PubMed] [Google Scholar]
- Shearer G. M., Rabson A. S. Semen and AIDS. Nature. 1984 Mar 15;308(5956):230–230. doi: 10.1038/308230a0. [DOI] [PubMed] [Google Scholar]
- Stankova L., Drach G. W., Hicks T., Zukoski C. F., Chvapil M. Regulation of some functions of granulocytes by zinc of the prostatic fluid and prostate tissue. J Lab Clin Med. 1976 Oct;88(4):640–648. [PubMed] [Google Scholar]
- Stites D. P., Erickson R. P. Suppressive effect of seminal plasma on lymphocyte activation. Nature. 1975 Feb 27;253(5494):727–729. doi: 10.1038/253727a0. [DOI] [PubMed] [Google Scholar]
- Williamson J. D. Semen polyamines in AIDS pathogenesis. Nature. 1984 Jul 12;310(5973):103–103. doi: 10.1038/310103a0. [DOI] [PubMed] [Google Scholar]


