Abstract
Malignant rabbit fibroma virus (MV) directly suppresses generation of antibody responses and mitogen induced T and B lymphocyte proliferation. We investigated whether this phenomenon required expression of the complete viral genome. Phosphonoacetic acid (PAA) inhibits poxvirus specific DNA polymerases. Adding PAA to cultures reduces both MV replication and mitogen-driven rabbit lymphocyte proliferation in a dose-dependent fashion. A dose of PAA adequate to inhibit MV replication by about 97%, but insufficient to reduce lymphocyte proliferation appreciably, does not affect the ability of MV to suppress lymphocyte proliferation or initiation of antibody production. Spleen cells from MV tumour-bearing rabbits contain very little virus, but inhibit the proliferative and antibody forming responses of normal spleen cells. This activity is shown here to reflect the production by T lymphocytes of a soluble mediator of greater than 25 kD molecular weight. Adding PAA to these mixed spleen cell cultures does not alter the ability of MV to induce T suppressor activity in host lymphocytes. Thus, these immunosuppressive capabilities of MV appear to reflect early MV gene functions.
Full text
PDF



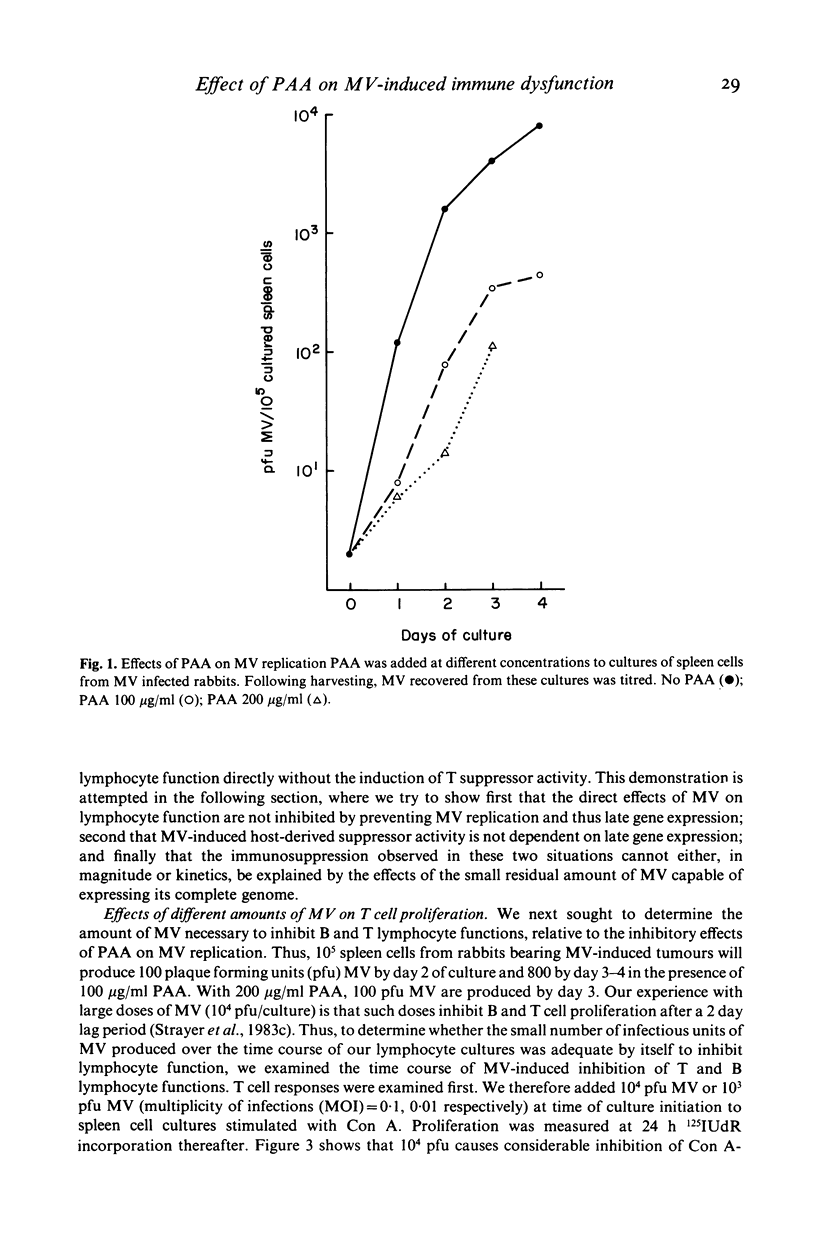
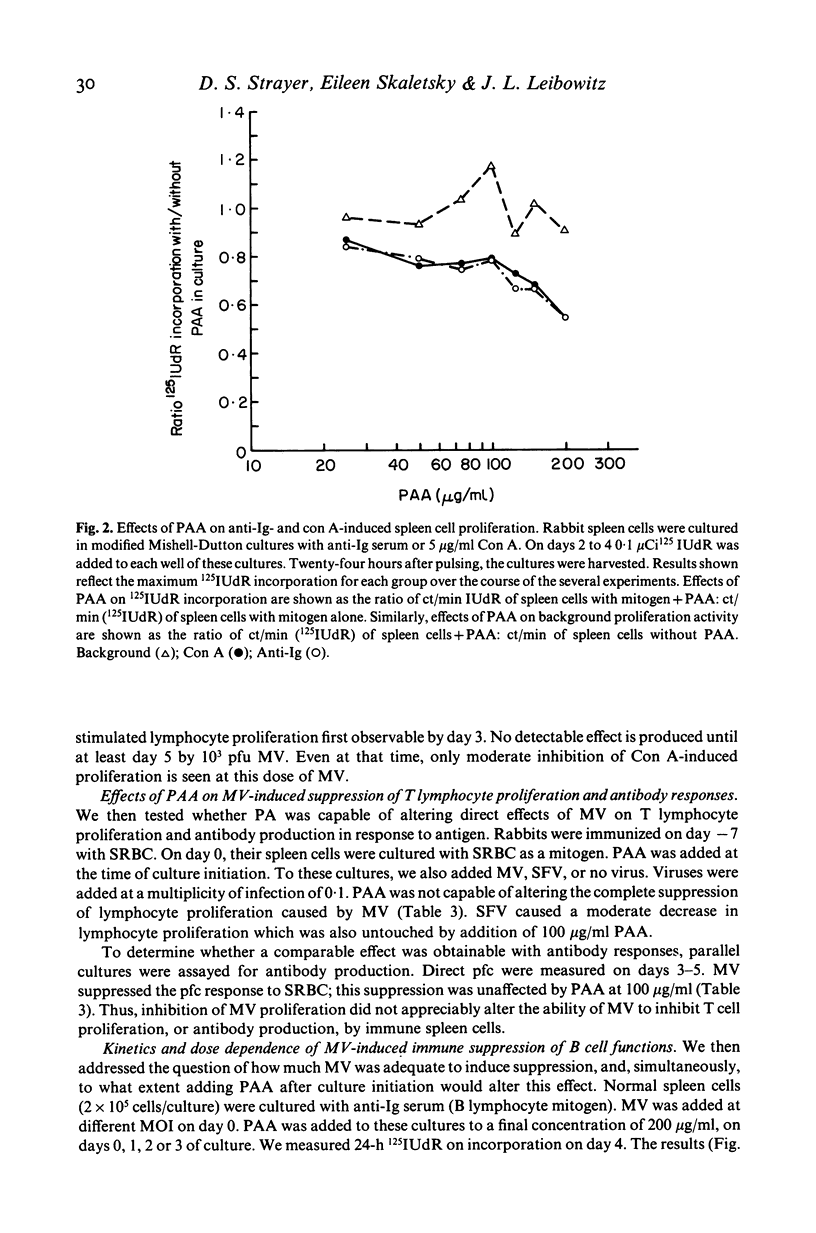
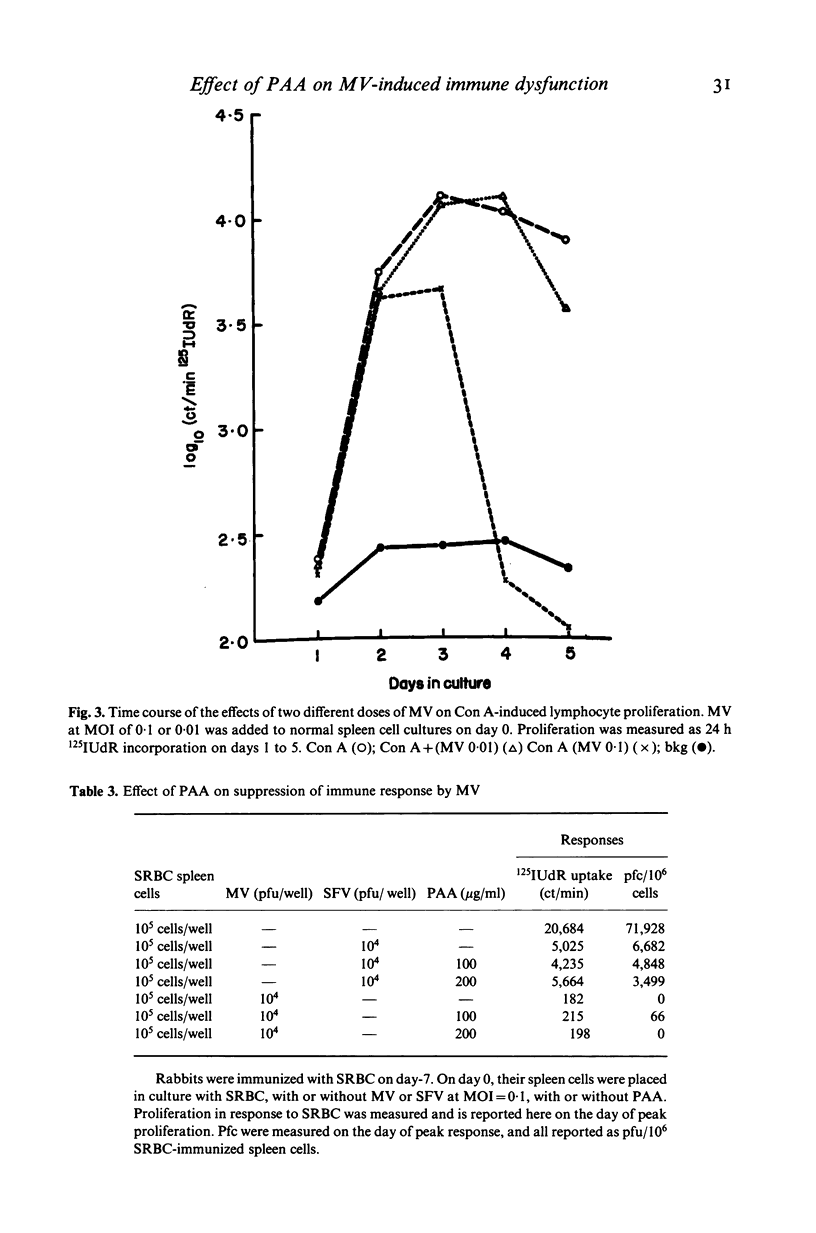
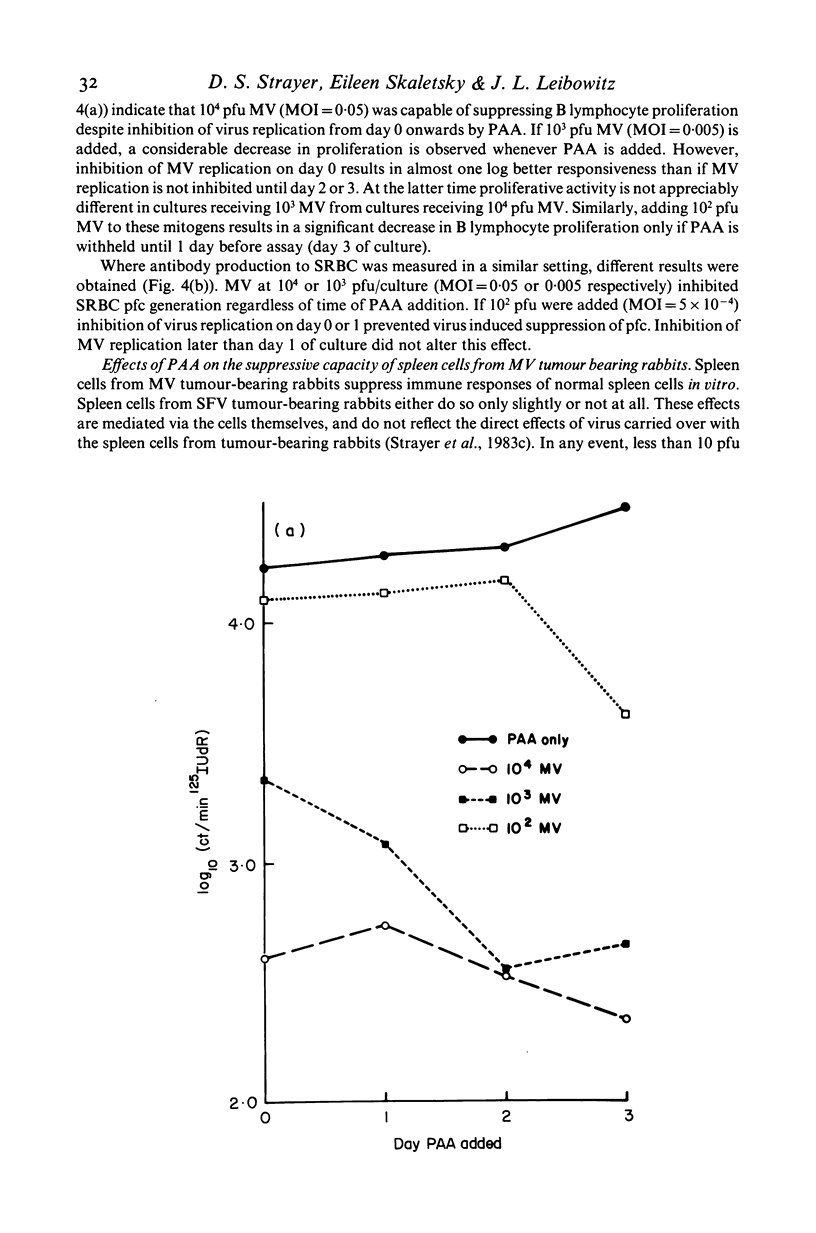
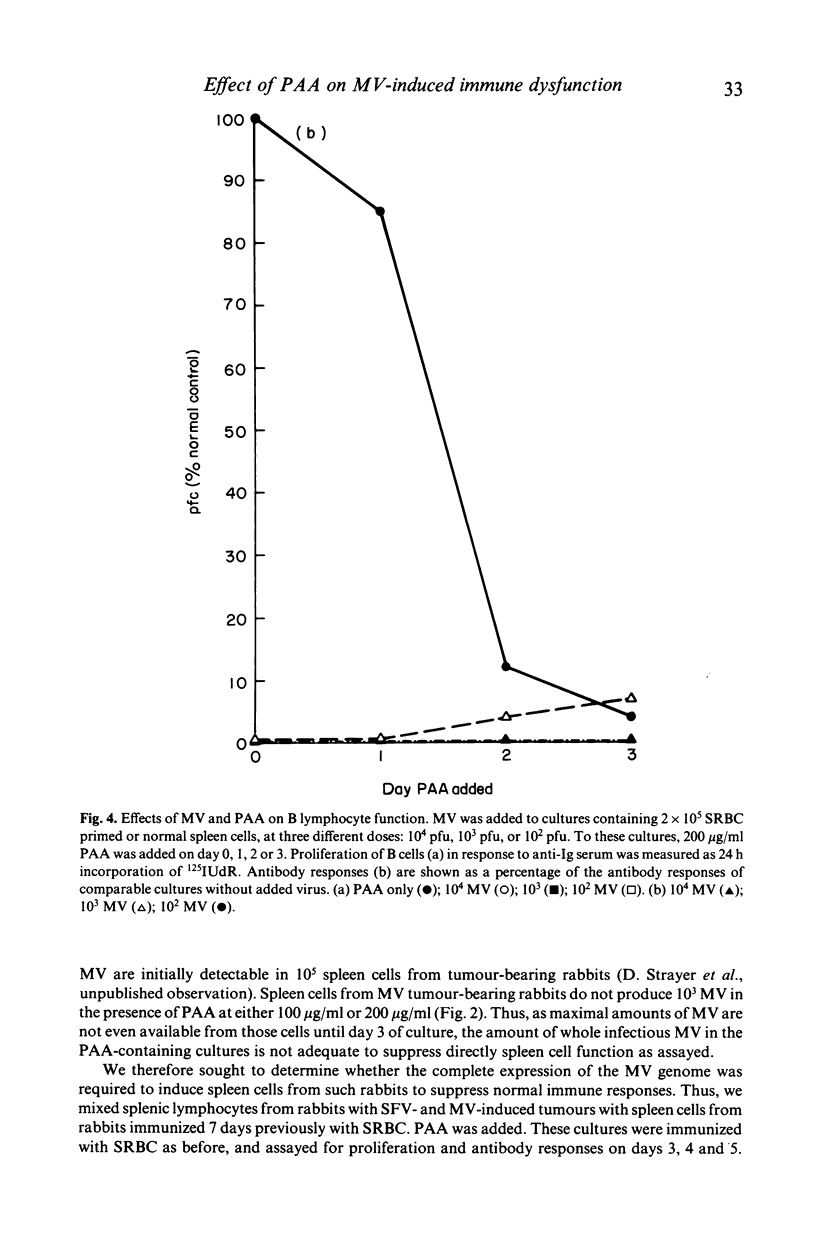
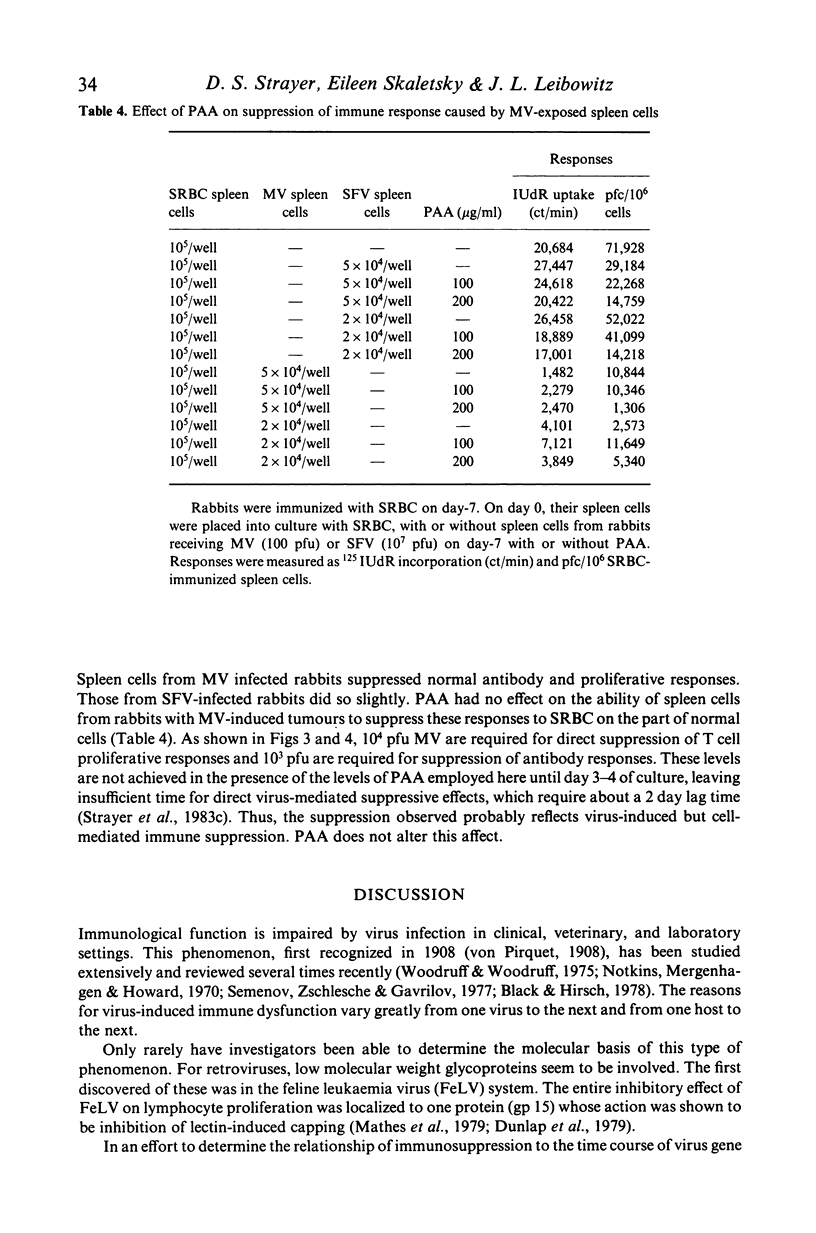


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Block W., Upton C., McFadden G. Tumorigenic poxviruses: genomic organization of malignant rabbit virus, a recombinant between Shope fibroma virus and myxoma virus. Virology. 1985 Jan 15;140(1):113–124. doi: 10.1016/0042-6822(85)90450-7. [DOI] [PubMed] [Google Scholar]
- Corbeil L. B., Strayer D. S., Skaletsky E., Wunderlich A., Sell S. Immunity to pasteurellosis in compromised rabbits. Am J Vet Res. 1983 May;44(5):845–850. [PubMed] [Google Scholar]
- Dunlap J. E., Nichols W. S., Hebebrand L. C., Mathes L. E., Olsen R. G. Mobility of lymphocyte surface membrane concanavalin A receptors of normal and feline leukemia virus-infected viremic felines. Cancer Res. 1979 Mar;39(3):956–958. [PubMed] [Google Scholar]
- Hobby G. L. Rifampin, a powerful new antimicrobial agent. J Infect Dis. 1969 Feb;119(2):195–196. doi: 10.1093/infdis/119.2.195. [DOI] [PubMed] [Google Scholar]
- JERNE N. K., NORDIN A. A. Plaque formation in agar by single antibody-producing cells. Science. 1963 Apr 26;140(3565):405–405. [PubMed] [Google Scholar]
- Mathes L. E., Olsen R. G., Hebebrand L. C., Hoover E. A., Schaller J. P., Adams P. W., Nichols W. S. Immunosuppressive properties of a virion polypeptide, a 15,000-dalton protein, from feline leukemia virus. Cancer Res. 1979 Mar;39(3):950–955. [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Howard R. J. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. 1970;24:525–538. doi: 10.1146/annurev.mi.24.100170.002521. [DOI] [PubMed] [Google Scholar]
- Overby L. R., Duff R. G., Mao J. C. Antiviral potential of phosphonoacetic acid. Ann N Y Acad Sci. 1977 Mar 4;284:310–320. doi: 10.1111/j.1749-6632.1977.tb21966.x. [DOI] [PubMed] [Google Scholar]
- PADGETT B. L., MOORE M. S., WALKER D. L. Plaque assays for myxoma and fibroma viruses and differentiation of the viruses by plaque form. Virology. 1962 Jul;17:462–469. doi: 10.1016/0042-6822(62)90141-1. [DOI] [PubMed] [Google Scholar]
- Redelman D., Scott C. B., Sheppard H. W., Jr, Sell S. In vitro studies of the rabbit immune system: II. Functional characterization of rabbit T and B populations separated by adherence to nylon wool or lysis with anti-thymocyte serum and complement. Cell Immunol. 1976 Jun 1;24(1):11–23. doi: 10.1016/0008-8749(76)90127-1. [DOI] [PubMed] [Google Scholar]
- Skaletsky E., Sharp P. A., Sell S., Strayer D. S. Immunologic dysfunction during viral oncogenesis. II. Inhibition of cellular immunity to viral antigens by malignant rabbit fibroma virus. Cell Immunol. 1984 Jun;86(1):64–74. doi: 10.1016/0008-8749(84)90359-9. [DOI] [PubMed] [Google Scholar]
- Strayer D. S., Cabirac G., Sell S., Leibowitz J. L. Malignant rabbit fibroma virus: observations on the culture and histopathologic characteristics of a new virus-induced rabbit tumor. J Natl Cancer Inst. 1983 Jul;71(1):91–104. [PubMed] [Google Scholar]
- Strayer D. S., Sell S. Immunohistology of malignant rabbit fibroma virus--a comparative study with rabbit myxoma virus. J Natl Cancer Inst. 1983 Jul;71(1):105–116. [PubMed] [Google Scholar]
- Strayer D. S., Sell S., Skaletsky E., Leibowitz J. L. Immunologic dysfunction during viral oncogenesis. I. Nonspecific immunosuppression caused by malignant rabbit fibroma virus. J Immunol. 1983 Nov;131(5):2595–2600. [PubMed] [Google Scholar]
- Strayer D. S., Skaletsky E., Cabirac G. F., Sharp P. A., Corbeil L. B., Sell S., Leibowitz J. L. Malignant rabbit fibroma virus causes secondary immunosuppression in rabbits. J Immunol. 1983 Jan;130(1):399–404. [PubMed] [Google Scholar]
- Strayer D. S., Skaletsky E., Leibowitz J. L. In vitro growth of two related leporipoxviruses in lymphoid cells. Virology. 1985 Sep;145(2):330–334. doi: 10.1016/0042-6822(85)90167-9. [DOI] [PubMed] [Google Scholar]
- VERNA J. E., EYLAR O. R. Rabbit fibroma virus plaque assay and in vitro studies. Virology. 1962 Oct;18:266–273. doi: 10.1016/0042-6822(62)90013-2. [DOI] [PubMed] [Google Scholar]


