Abstract
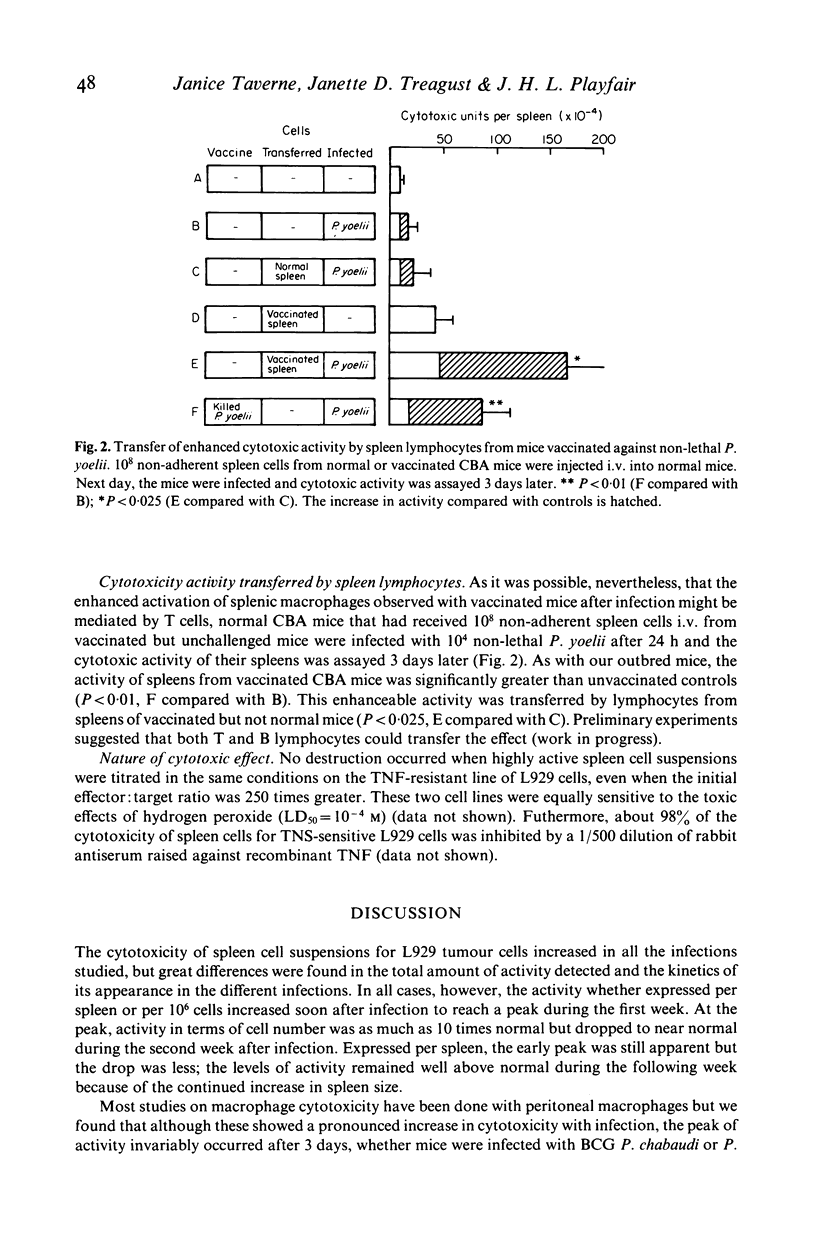
We investigated the development of cell-mediated immunity in lethal and non-lethal malarial infections by assaying the cytotoxic activity of spleen cells for L929 tumour cells at different times after infection of mice with the lethal P. berghei, a lethal variant of Plasmodium yoelii and the non-lethal P. yoelii and P. chabaudi. In all cases the cytotoxicity increased to a peak during the first week and then diminished but the time of the peak varied with the infection; its activity was lowest with P. berghei. A second peak occurred in the non-lethal infections at the time of recovery. A protective vaccine accelerated and enhanced the early peak of cytotoxicity. The activity was mediated by adherent phagocytic cells, probably through the release of tumour necrosis factor (TNF) by macrophages since it was inhibited by antiserum against recombinant mouse TNF and did not destroy TNF-resistant L929 cells. Its induction was not dependent on T cells since it occurred in T cell-deficient mice infected with non-lethal P. yoelii. However, the accelerated increase associated with vaccination could be adoptively transferred by spleen lymphocytes from vaccinated mice.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkmann V., Kaufmann S. H., Simon M. M., Fischer H. Role of macrophages in malaria: O2 metabolite production and phagocytosis by splenic macrophages during lethal Plasmodium berghei and self-limiting Plasmodium yoelii infection in mice. Infect Immun. 1984 Jun;44(3):743–746. doi: 10.1128/iai.44.3.743-746.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Taffet S. M., Adams D. O. The relationship between competence for secretion of H2O2 and completion of tumor cytotoxicity by BCG-elicited murine macrophages. J Immunol. 1982 Apr;128(4):1781–1785. [PubMed] [Google Scholar]
- Cox F. E. Differential susceptibility of rodent malaria parasites to nonspecific immunity. Bull Soc Pathol Exot Filiales. 1983 Nov;76(5):503–508. [PubMed] [Google Scholar]
- Dockrell H. M., Alavi A., Playfair J. H. Changes in oxidative burst capacity during murine malaria and the effect of vaccination. Clin Exp Immunol. 1986 Oct;66(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- Dockrell H. M., Playfair J. H. Killing of blood-stage murine malaria parasites by hydrogen peroxide. Infect Immun. 1983 Jan;39(1):456–459. doi: 10.1128/iai.39.1.456-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch H., Gifford G. E. In vitro production of rabbit macrophage tumor cell cytotoxin. Int J Cancer. 1983 Jul 15;32(1):105–112. doi: 10.1002/ijc.2910320117. [DOI] [PubMed] [Google Scholar]
- Grosskinsky C. M., Ezekowitz R. A., Berton G., Gordon S., Askonas B. A. Macrophage activation in murine African trypanosomiasis. Infect Immun. 1983 Mar;39(3):1080–1086. doi: 10.1128/iai.39.3.1080-1086.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidaris C. G., Haynes J. D., Meltzer M. S., Allison A. C. Serum containing tumor necrosis factor is cytotoxic for the human malaria parasite Plasmodium falciparum. Infect Immun. 1983 Oct;42(1):385–393. doi: 10.1128/iai.42.1.385-393.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haranaka K., Satomi N., Sakurai A. Differences in tumour necrosis factor productive ability among rodents. Br J Cancer. 1984 Oct;50(4):471–478. doi: 10.1038/bjc.1984.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelchuk R., Dockrell H. M., Playfair J. H. T-independent macrophage changes in murine malaria. Clin Exp Immunol. 1983 Mar;51(3):487–493. [PMC free article] [PubMed] [Google Scholar]
- Matthews N., Watkins J. F. Tumour-necrosis factor from the rabbit. I. Mode of action, specificity and physicochemical properties. Br J Cancer. 1978 Aug;38(2):302–309. doi: 10.1038/bjc.1978.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Falk W., Meltzer M. S. Inhibition of nonspecific tumoricidal activity by activated macrophages with antiserum against a soluble cytotoxic factor. Infect Immun. 1981 Jul;33(1):156–164. doi: 10.1128/iai.33.1.156-164.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Shear H. L. Oxidative killing of the intraerythrocytic malaria parasite Plasmodium yoelii by activated macrophages. J Immunol. 1984 Jan;132(1):424–431. [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Cottrell B. J. Protection of mice against malaria by a killed vaccine: differences in effectiveness against P. yoelii and P. berghei. Immunology. 1977 Oct;33(4):507–515. [PMC free article] [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Dockrell H. M., Agomo P. U., Taverne J. Cell-mediated immunity in the liver of mice vaccinated against malaria. Nature. 1979 Dec 13;282(5740):731–734. doi: 10.1038/282731a0. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. Immunity to malaria. Br Med Bull. 1982 May;38(2):153–159. doi: 10.1093/oxfordjournals.bmb.a071752. [DOI] [PubMed] [Google Scholar]
- Playfair J. H., de Souza J. B. Lymphocyte traffic and lymphocyte destruction in murine malaria. Immunology. 1982 May;46(1):125–133. [PMC free article] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. Splenomegaly, enhanced phagocytosis, and anemia are thymus-dependent responses to malaria. Infect Immun. 1978 Jun;20(3):728–731. doi: 10.1128/iai.20.3.728-731.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., James S., Sher A. The respiratory burst is not required for killing of intracellular and extracellular parasites by a lymphokine-activated macrophage cell line. Eur J Immunol. 1985 Jun;15(6):553–558. doi: 10.1002/eji.1830150605. [DOI] [PubMed] [Google Scholar]
- Strickland G. T., Ahmed A., Sayles P. C., Hunter K. W. Murine malaria: cellular interactions in the immune response. Am J Trop Med Hyg. 1983 Nov;32(6):1229–1235. doi: 10.4269/ajtmh.1983.32.1229. [DOI] [PubMed] [Google Scholar]
- Taverne J., Depledge P., Playfair J. H. Differential sensitivity in vivo of lethal and nonlethal malarial parasites to endotoxin-induced serum factor. Infect Immun. 1982 Sep;37(3):927–934. doi: 10.1128/iai.37.3.927-934.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Dockrell H. M., Playfair J. H. Endotoxin-induced serum factor kills malarial parasites in vitro. Infect Immun. 1981 Jul;33(1):83–89. doi: 10.1128/iai.33.1.83-89.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Matthews N., Depledge P., Playfair J. H. Malarial parasites and tumour cells are killed by the same component of tumour necrosis serum. Clin Exp Immunol. 1984 Aug;57(2):293–300. [PMC free article] [PubMed] [Google Scholar]
- Waki S., Nakazawa S., Taverne J., Targett G. A., Playfair J. H. Immunity to an attenuated variant of Plasmodium berghei: role of some non-specific factors. Parasitology. 1985 Oct;91(Pt 2):263–272. doi: 10.1017/s0031182000057358. [DOI] [PubMed] [Google Scholar]
- Weidanz W. P., Grun J. L. Antibody-independent mechanisms in the development of acquired immunity to malaria. Adv Exp Med Biol. 1983;162:409–423. doi: 10.1007/978-1-4684-4481-0_37. [DOI] [PubMed] [Google Scholar]
- Wozencraft A. O., Dockrell H. M., Taverne J., Targett G. A., Playfair J. H. Killing of human malaria parasites by macrophage secretory products. Infect Immun. 1984 Feb;43(2):664–669. doi: 10.1128/iai.43.2.664-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


