Abstract
Dormancy and subsequent regrowth of adventitious buds is a critical physiological process for many perennial plants. We have used the expression of hormone and cell cycle-responsive genes as markers to follow this process in leafy spurge (Euphorbia esula). In conjunction with earlier studies, we show that loss of mature leaves results in decreased sugar levels and increased gibberellin perception in underground adventitious buds. Gibberellin is sufficient for induction of S phase-specific but not M phase-specific gene expression. Loss of both apical and axillary buds or inhibition of polar auxin transport did not result in induction of S phase- or M phase-specific gene expression. Loss of polar auxin transport was necessary for continuation of the cell cycle and further bud development if the S phase was previously initiated.
Leafy spurge (Euphorbia esula) is a deep-rooted perennial weed that primarily infests range and recreational lands in the northern Great Plains of the United States and Canada. Like many perennial weeds, leafy spurge propagates through the production and growth of numerous underground adventitious buds. Once formed, these buds enter a quiescent state and remain dormant until growth is re-initiated by separation from, or death of, the aerial portion of the plant.
Little is known about the molecular mechanisms that control dormancy and growth of underground buds and seeds in leafy spurge or other perennial plants. However, physiological studies have shown that correlative inhibition of leafy spurge underground buds is maintained by at least two separate signals. One signal is produced in the shoot apices (young expanding leaves and meristems) of the plant and likely is connected to auxin perception (Horvath, 1999). The other signal is produced in the mature leaves, requires photosynthesis for its production and/or transport, and can be overcome by application of gibberellic acid (GA3; Horvath, 1999). This second signal is likely to be a sugar (Chao et al., 2000).
It is not known how these physiological signals control the growth of underground buds. There is some evidence that auxin acts indirectly through products of the Rms genes to inhibit growth of buds below the apical meristem (Beveridge et al., 2000). How this interaction functions to prevent growth of axillary and adventitious buds is not known. Interestingly, auxin appears to play a positive role during bud growth. It has been shown that auxin levels increase in growing bean (Phaseolus vulgaris) buds (Gocal et al., 1991). Also, inhibition of auxin-repressible gene expression suggests that auxin perception within the quiescent axillary buds of pea increases concomitantly with resumed growth (Stafstrom et al., 1998). Auxin has been shown to play an important role in regulating the level of key cell cycle components in plants (Ruegger et al., 1998).
The role of sugar in the control of growth and development is just beginning to be understood. Sugar is known to play a key role in several different signaling pathways including photomorphogenesis, cell growth, and stress responses (Jang et al., 1997; Ho et al., 2001). Both sugar and cytokinins have been shown to induce G1 cyclin expression in tissue culture (Gaudin et al., 2000; Soni et al., 1995). Also, sugar has been shown to inhibit gibberellin signaling in several different plant systems (Perata et al., 1997; Xu et al., 1998; Chao et al., 2000). Gibberellin is suspected of playing a key role in the control of cell division and elongation and in para-dormancy phenomenon (also known as apical dominance or correlative inhibition; Cline, 1991; Sauter et al., 1995; Gendreau et al., 1999). Thus, it seems likely that sugar may, in part, be affecting cell cycle control through its interactions with gibberellin. Additional supporting evidence for an interaction of sugar with gibberellin signaling comes from recent findings that several sugar-insensitive mutants are allelic with genes known to play a role in abscisic acid (ABA) signal transduction (Huijser et al., 2000; Laby et al., 2000). Antagonistic cross-talk between ABA and gibberellin signaling is a well-known phenomenon.
To better understand how these signals control growth in leafy spurge, we have undertaken the cloning of genes that are differentially expressed concomitantly with the initiation of underground bud growth. Such differentially expressed genes are likely to contain cis-acting elements within their regulatory sequences that interact with components of the signaling process controlling growth and dormancy. Identifying such sequences and the proteins that interact with them will be critical for deciphering the signaling pathways involved in controlling their expression and will likely provide clues as to the signaling pathways controlling growth and dormancy in underground buds of leafy spurge. In this paper, we demonstrate the differential expression of a number of genes likely to be controlled by gibberellin, cell cycle, and developmental processes. This information is used to develop a model for controlling initiation of underground bud growth.
RESULTS
Identification of Differentially Expressed Genes
Previous studies indicating changes in gene expression (Anderson and Horvath, 2001) and cell cycle activity (Horvath and Anderson, 2000) were initiated between 36 to 48 h after defoliation (removal of all above ground foliar tissue). From approximately 2,000 random expressed sequence tags derived from a cDNA library of 3-d-induced buds from leafy spurge, we identified at least three that were likely to serve as indicators of changes in cell cycle activity or hormone levels. These genes include a Histone H3 gene, Tubulin, and a GA-responsive gene (EeGASA4) that is 63% identical in amino acid sequence to Gasa4 from Arabidopsis. Histone H3 is known to be specifically up-regulated during S phase of the cell cycle and is a commonly used marker for initiation of cell division (Fobert et al., 1994). Tubulin is known to be up-regulated in late G2 phase of the cell cycle and has also served as a marker for this phase of cell division (Vantard et al., 2000). GASA4 gene expression has been well characterized in Arabidopsis (Aubert et al., 1998). GASA4 is expressed preferentially in floral buds and in growing tissues such as meristems and germinating seeds and is known to be specifically up-regulated by exogenous GA3 in these tissues (Aubert et al., 1998). Northern-blot analysis indicated that all of these genes were consistently differentially regulated to some degree in the experiments described below and are expressed preferentially in growing tissues consistent with their putative identity.
Tissue Specificity and Stress Responses
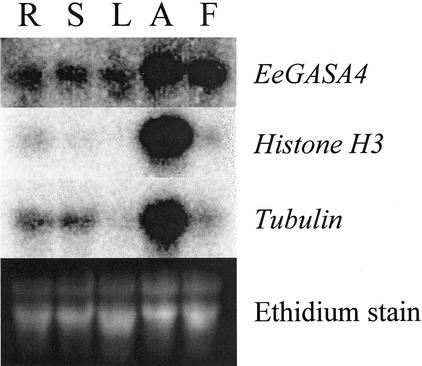
Most genes used for these studies have not been well characterized in leafy spurge. To better understand the expression pattern of these genes, RNA was collected from various plant tissues, and the resulting RNA was subjected to northern-blot analysis (Fig. 1). Results of these experiments show that Histone H3, Tubulin, and EeGASA4 all show the highest levels of expression in growing shoot apices of leafy spurge. In tissues with fewer dividing cells such as roots, stems, and leaves, all three genes are expressed at reduced levels relative to their expression in the shoot apices. Like GASA4 of Arabidopsis, EeGASA4 is preferentially expressed in flowers and in the shoot apices.
Figure 1.
Northern blots of RNA from mature roots (R), mature stems (S), mature leaves (L), shoot apices (A), and flowers (F). Blots were hybridized to the designated clones EeGASA4 (accession no. AW862634), Histone H3 (accession no. AF239930), and Tubulin (accession no. AW832663) and visualized on a Packard Instant Imager.
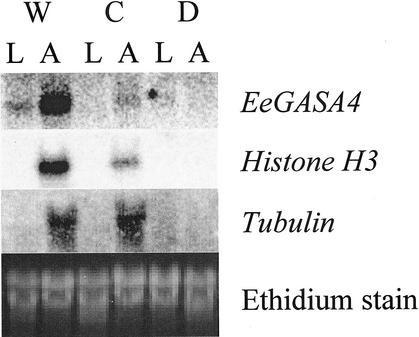
To better understand the role of these genes in the growth response, these genes were used to probe northern blots of RNA collected either from mature leaves or shoot apices of plants subjected to various growth-inhibiting stresses (Fig. 2). Results from these experiments show that Histone H3 and EeGASA4 are significantly down-regulated under both cold and drought stress in the shoot apices of leafy spurge. Tubulin gene expression was not significantly down-regulated by cold but was down-regulated by drought stress.
Figure 2.
Northern blot of shoot apices (A) and mature leaves (L) from control (W), cold-stressed (C), or drought-stressed (D) plants. Blots were hybridized to the designated clones and visualized on a Packard Instant Imager.
Temporal Expression of Differentially Expressed Genes
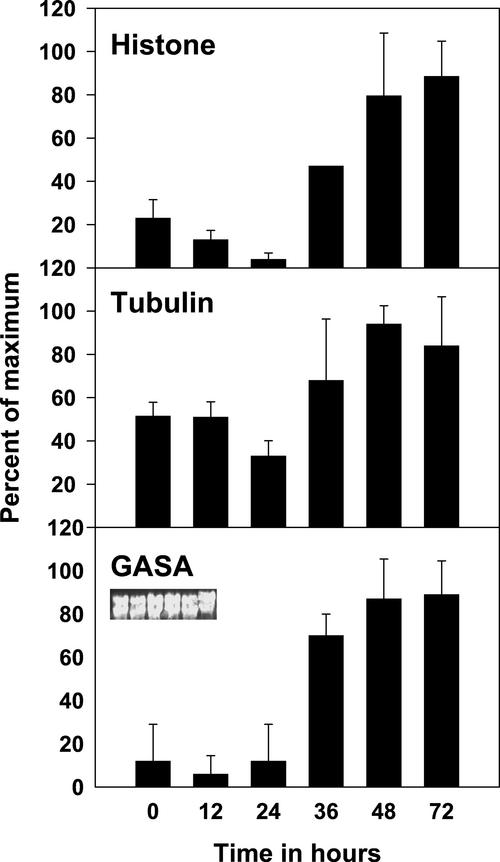
Clones of the three genes were used to probe northern blots of RNA from underground buds collected at various times after defoliation. All the clones showed reproducible increases in expression between 24 and 72 h after defoliation (Fig. 3). It is interesting to note that EeGASA4 appeared to be up-regulated concomitantly with Histone H3 between 24 and 36 h after defoliation. Increased expression of Tubulin was only consistent at 48 and 72 h after defoliation.
Figure 3.
Histograms representing two separate northern blots of RNA from underground buds harvested at various times after defoliation (0–72 h) expressed as a percentage of the maximum signal per hybridization. Error bars represent variance between two experiments. Inset shows ethidium bromide staining for one of the sets as a loading control. Blots were hybridized to the designated clones and quantified on a Packard Instant Imager.
Expression of Differentially Expressed Genes after Removal of Leaves or Apices
Previous studies have shown that two separate signals control underground bud growth. One is leaf derived (and is likely a sugar), and the other is apices derived (and is likely auxin; Chao et al., 2000; Horvath, 1999). To determine whether expression of selected genes could provide clues as to the molecular action of these two signals, RNA was extracted from underground buds collected 3 d after removal of the shoot apices by itself (meristemless) or in combination with removal of leaves (leafless) or axillary buds (budless). The resulting RNA was probed with the selected clones (Fig. 4). Histone H3 and EeGASA4 showed a consistent increase in expression after 3 d in leafless, but not in meristemless or budless plants. It is important to note that no growth in underground buds is detected even 2 weeks after treatment in meristemless, leafless or budless plants. Only full defoliation results in growth of underground buds. Tubulin was not induced by any of the treatments except complete defoliation.
Figure 4.
Schematic of plant treatments and northern blot of RNA from underground buds from controls (0) and from underground buds harvested 3 d after defoliation (3), 3 d after excision of apical meristem (m), after excision of apical meristem and axillary buds (b), and excision of apical meristem and leaves (l). Blots were hybridized to the designated clones and visualized on a Packard Instant Imager.
GA3 But Not Loss of Polar Auxin Transport Induces S Phase
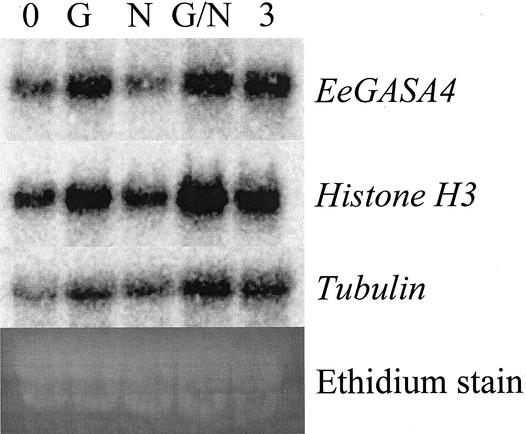
Previous studies have lead us to hypothesize that the leaf-derived signal acts through GA3 signaling to induce Histone H3 and EeGASA4 and that the apices-derived signal requires polar auxin transport to inhibit underground bud growth in leafy spurge. To substantiate this hypothesis, plants were watered with a GA3 solution or treated with a paste of the auxin transport inhibitor N-1-naphthylphthalamic acid (NPA) or both, and the expression of the selected genes was determined for underground buds collected 3 d after treatments (Fig. 5). The results from these experiments clearly show that Histone H3 and EeGASA4 expression is induced by GA3 treatment but not by loss of polar auxin transport. Tubulin expression was only substantially up-regulated after both loss of auxin transport and addition of GA3. These data indicate that GA3 and NPA treatments result in patterns of gene expression similar to those induced by loss the leaf- and apices-derived signals, respectively.
Figure 5.
Northern blot of RNA from underground buds from controls (0), underground buds harvested 3 d after application of GA3 (G), NPA (N), GA3 plus NPA (G/N), or defoliation (3). Blots were hybridized to the designated clones and visualized on a Packard Instant Imager.
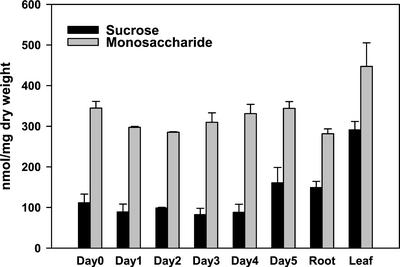
Sugar Levels in Underground Buds Drop within 24 h of Defoliation
Suc and Glc levels in underground buds of leafy spurge were determined at various times after defoliation (Fig. 6). The results showed that underground buds of intact plants contained relatively higher levels of mono- and disaccharide than the buds harvested 1 to 2 d after defoliation. Suc levels decreased in the first 3 d after defoliation and began to increase above control levels by 5 d. Monosaccharide levels were significantly lower than controls within 24 h after defoliation. Monosaccharide levels continued to decrease for the first 2 d and began to elevate from the 3rd d. Increases in sugar levels the 3rd d after defoliation may indicate an active cellular action at the meristematic region. The result also showed that monosaccharide levels were consistently 1- to 2-fold higher than that of Suc, suggesting that Suc might be quickly hydrolyzed after it was imported into the bud cells. Sugar levels in leaves and roots are included to provide an indication of the relative sugar content in source and sink organs, respectively.
Figure 6.
Suc (black bars) and monosaccharide (gray bars) levels in underground buds of leafy spurge from two separate experiments at various times after defoliation. Sugar levels for sink (roots) and source (leaves) tissues are included. Error bars represent sd.
DISCUSSION
Temporal Expression of Histone H3 and Tubulin Suggests That Growth of Underground Buds Is Not Initiated prior to 12 h after Defoliation
In this paper, we have shown that genes responsive to cell cycle and gibberellin signals are differentially regulated in growing versus non-growing tissues of leafy spurge. Not surprisingly, genes that are differentially expressed in other plant systems in response to cell division such as Histone H3 and Tubulin are consistently up-regulated in underground buds between 24 to 36 h and 36 to 48 h, respectively, after growth induction by defoliation. This time corresponds well with previous studies on biochemical changes associated with increased cell cycle activity in underground buds of leafy spurge (Horvath and Anderson, 2000) and observations of increased numbers of mitotic figures in the underground bud leaf primordia within 3 d after defoliation (data not shown). Also, the possible delay in expression of Tubulin compared with Histone H3 is consistent with previous studies indicating that Tubulin is required in late G2/M, whereas Histone H3 is required in S phase (Fobert et al., 1994; Vantard et al., 2000). As a consequence, these genes serve as excellent indicators of growth induction in underground buds before any visible indication of growth. Similar studies have been used to follow progression of the cell cycle in other plant systems (Devitt and Stafstrom, 1995; Sauter et al., 1995).
The induction of Histone H3 indicates that S phase is not initiated earlier than 24 to 36 h in underground buds. Similar studies following the timing and duration of the cell cycle in peas after removal of apical dominance suggests no more than 12 h are required to complete preparation for the G1/S phase transition to occur (Devitt and Stafstrom, 1995). Unless preparation for G1/S transition is unusually slow in underground buds of leafy spurge or is controlled differently from most other previously studied systems, induction of genes needed for initiation of growth, such as D-class cyclins, likely occurs 12 h or more after defoliation. Efforts are under way to isolate D class cyclins from leafy spurge to test this hypothesis.
Sugar Levels Likely Control Gibberellin Perception and Subsequent Growth Induction
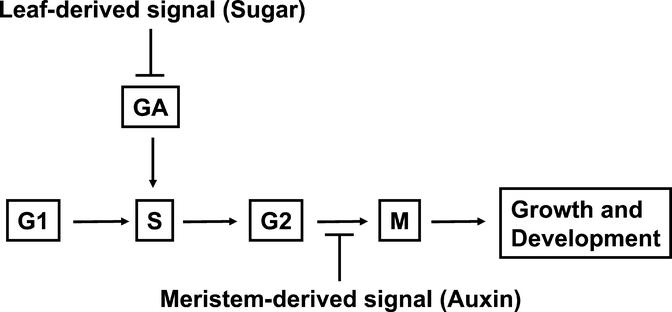
We hypothesize that sugar produced in photosynthesizing leaves inhibits underground bud growth in leafy spurge by negatively regulating the production and/or signal transduction of gibberellin. Previous experiments indicated that photosynthesizing leaves or exogenous sugar was sufficient for inhibiting bud growth (Horvath, 1999; Chao et al., 2000). Watering plants with GA3 could overcome the leaf-derived signal, but could not overcome the apices-derived signal (Horvath, 1999). Gibberellin was necessary but not sufficient for induction of bud growth (Chao et al., 2001). Application of GA3 is sufficient to induce both genes in underground buds of leafy spurge (Fig. 5). EeGASA4 is clearly up-regulated by GA3 in leafy spurge, and EeGASA4 orthologs are known to be specifically up-regulated by GA3 in tomato (Lycopersicon esculentum) and Arabidopsis (Aubert et al., 1998; Shi and Olszewski, 1998). Previous studies demonstrated that Histone H3 is specifically induced in S phase of the cell cycle in other plants (Fobert et al., 1994) and is almost certainly up-regulated specifically during S phase of the cell cycle in spurge. Both EeGASA4 and Histone H3 show concomitant increases in expression between 24 and 36 h after the underground buds are released from dormancy (Fig. 3). Sugar levels in the buds fall before this time (Fig. 6). Cell cycle control by gibberellin was shown in several other plant systems. Examples include gibberellin-induced endoreduplication of DNA in Arabidopsis (Gendreau et al., 1999) and gibberellin requirement for expression of Histone genes in deep water rice internodes (Sauter et al., 1995). Combined, these data are consistent with our hypothesis that sugar regulates gibberellin signal transduction and that gibberellin, in turn, has some effect on initiation of S phase in underground buds of leafy spurge (Fig. 7).
Figure 7.
Diagram showing hypothesized signaling pathways and their interaction with the cell cycle. Sugar inhibits gibberellin signaling required for induction of S phase. Auxin inhibits the cell cycle at the G2/M restriction point.
There is also evidence for cross-talk between sugar and gibberellin signaling pathways controlling growth and development in leafy spurge and other plants (Perata et al., 1997; Xu et al., 1998; Chao et al., 2000). Sugar is also known to influence ABA responses in some Arabidopsis tissues (Huijser et al., 2000; Laby et al., 2000). ABA levels are important in seed dormancy and in the dormancy of adventitious buds of many trees (Debeaujon and Koornneef, 2000; Rohde et al., 2000). Gibberellin has been shown to inhibit ABA signaling in seeds (White and Rivin, 2000), and general antagonism between ABA and gibberellin signaling is well known. It is also interesting to note that a cyclin-dependent kinase inhibitor (Ick1), capable of reducing the rate of cell division, is induced by ABA in Arabidopsis (Wang et al., 1998). Because gibberellin is known to be antagonistic to ABA signaling in many systems, it will be interesting to determine whether gibberellin control of cell division in underground buds of leafy spurge is acting, at least in part, through suppression of Ick1 gene expression.
Sugar and Auxin Act at Different Points in the Cell Cycle to Control Underground Bud Growth and Development
The expression pattern of these genes provides an indication for the mode of action for these signals (Fig. 7). Previous studies demonstrated that growth of underground buds is not induced after removal of either the sugar or auxin source alone and that auxin or sugar can inhibit greening and significantly prevent dry weight accumulation in underground bud growth (Horvath, 1998, 1999; Chao et al., 2000). Yet, Histone H3 and EeGASA4 expression was consistently induced after removal of the mature leaves of the plant but not by removal of the major auxin-producing organs. Also, application of GA3 but not treatments that block auxin transport resulted in increased Histone H3 and EeGASA4 expression. This suggests that loss of mature leaves and subsequent reduction in leaf-derived sugar overcomes the G1/S restriction point and indicates that GA3 application is sufficient for induction of S phase but not induction of M phase and/or continued growth and development in underground buds of leafy spurge. Continued failure of growth in the presence of the auxin-producing organs suggests that auxin is responsible for maintaining inhibition of growth at a later point in the cell cycle.
Tubulin expression does not reach the levels observed in underground buds 3 d after defoliation unless both the sugar and auxin signals are removed by excision of leaves, shoot apices, and axillary buds or if both these signals are circumvented by application of GA3 and NPA. The lack of Tubulin induction in leafless or budless plants suggests that the loss of either the auxin-producing organs or the sugar-producing organs alone does not result in induction of G2/M. Thus, Tubulin expression requires loss of inhibitory signals produced by both of these organs or production of secondary signals generated by loss of both apical auxin and sugar. However, the observed lack of Tubulin gene expression is limited to underground buds from plants that are less than 4 months old. Underground buds collected from older plants appeared to be less subject to inhibition by the apices-derived signals. In very old plants (>5 months old), GA3 treatment is often sufficient for induction of underground bud growth (D.P. Horvath and W.S. Chao, unpublished data).
Growth-Inhibiting Stresses Reduce Expression of Gibberellin and Cell Cycle-Controlled Genes
All of the genes studied are up-regulated in actively growing tissues such as shoot apices (Fig. 1). Histone H3 and EeGASA4 are down-regulated in actively growing shoot apices after cold and drought stress (Fig. 2). Both cold stress and drought stress are known to increase levels of ABA (Chen et al., 1983). Expression of the cell cycle inhibitor Ick1 is known to be induced by ABA in Arabidopsis (Wang et al., 1998). Assuming leafy spurge has a functional ortholog of Ick1 and that it is responsive to the same signals that control it in Arabidopsis, it is possible that both of these stresses inhibit cell division before the G1/S transition (and Histone H3 expression), in part via ABA induction of the cyclin-dependent kinase inhibitor Ick1. Reduction in expression of EeGASA4 may be indicative of antagonistic actions of ABA on production or perception of gibberellin.
MATERIALS AND METHODS
Plant Material
Plants used for these experiments were started as shoot cuttings from a small group of plants originally isolated from a wild leafy spurge (Euphorbia esula) population in North Dakota (biotype 1984–ND001). Shoot cuttings were placed in Sunshine mix and grown in 1- × 8-inch cones in a greenhouse under an 18-h photoperiod at approximately 28°C±4°C for 3 months. Plants used for all of the studies above were single stems with 70 to 100 leaves and an average of 56 (sd = 20) root buds per plant. All harvested tissues were immediately frozen in liquid N2 and stored at −80°C until RNA extractions were initiated. All experiments were repeated with at least two separate sets of 14 to 21 individual plants treated at different times.
Plant Treatments
To study the temporal expression pattern of the genes, the crown and aerial portion of the plant was excised, and all underground buds greater then 0.25 mm in length were harvested at 12-h intervals. To reduce the possibility of observing circadian-regulated changes in gene expression, plants were excised so that harvest could be initiated between 8 am and 10 am daily.
To test the effects of drought and cold stress on gene expression, plants were placed in a growth chamber with a constant temperature of 5°C under an 18-h photoperiod. Mature leaves and shoot apices were harvested separately from 21 individual plants 5 to 6 d after initiation of cold stress. Alternatively, water was withheld from the plants until plants were wilted (5–7 d). Relative water content was measured at 78% and 64%, respectively, for two separate experiments. Again, mature leaves and shoot apices were harvested from 21 individual plants for RNA extraction.
To study the effects of various plant organs on underground bud growth, specific plant organs were removed as previously described (Horvath, 1999). In brief, plants were either left intact or had the entire aerial portion of the plant excised to the base of the crown. Alternatively, the top 10 cm of the plants was removed (meristemless), or/and they were then stripped of either mature leaves (leafless) or axillary buds (budless). All distinguishable underground buds 0.25 mm or larger were harvested 3 d after treatment.
To test the effects of hormones on gene expression in underground buds, plants were watered once with 25 mL of a 0.05% (v/v) Tween 20 solution with or without 1 mm GA3 (potassium salt) and on which the crown was abraded and ringed with lanolin paste that was or was not supplemented with 1% (w/v) NPA. All distinguishable underground buds 0.25 mm or larger were harvested 3 d after treatment.
Sugar Assays
Underground buds of 3-month-old leafy spurge plants were harvested into liquid N2 and lyophilized. Preweighed buds were placed into a 16- × 125-mm Pyrex tube containing 0.8 mL of 80% (v/v) ethanol at 100°C. After boiling for 20 min, another 0.8 mL of 80% (v/v) ethanol (100°C) was added and incubated for 20 min. The sample was transferred to a microfuge tube, and the ethanol was evaporated in a speed vacuum for 1 h. The supernatant was transferred into a clean tube, and the volume was adjusted to 450 μL.
Both monosaccharide and Suc were assayed by the Nelson-Somogyi method for reducing sugars (Chaplin, 1994). However, Suc assays required pretreatment with invertase (I-4504, Sigma, St. Louis) to release Glc and Fru. Invertase reaction was done by adding 5 μL of sample into a microfuge tube containing 95 μL of invertase solution (5 mg mL−1 in water and made immediately before use). To the mixture, 100 μL of 100 mm sodium acetate (pH 4.5) was added, mixed, and incubated at 55°C for 30 min. The whole reaction mixture (200 μL) was used for colorimetric analysis using the Nelson-Somogyi method. To assay monosaccharide, 10 μL of sample was diluted (1:20) and the diluent (200 μL) was used for colorimetric analysis.
Northern-Blot Analysis
RNA was collected using the Pine Tree Extraction method (Chang et al., 1993). Total RNA (50 μg) was separated on denaturing agarose gels and blotted according to standard techniques (Sambrook et al., 1989). DNA probes were prepared by PCR amplification of designated cDNAs followed by isolation of the resulting band after separation on agarose gels. Radiolabeled probes for the genes were prepared and hybridized to the various blots (5× SCC/50% [v/v] formamide) at 42°C overnight. Blots were washed four times in 2× SSC, 0.2% (w/v) SDS at room temperature for 5 min each and then twice at 65°C for 15 min each. The resulting hybridizations were visualized and/or quantified on a Packard Instant Imager. Linearity was maintained for all of the images presented. Genetic material used for generating probes for these studies were obtained from a leafy spurge expressed sequence tag database developed from a cDNA library made using underground buds harvested 3 d after defoliation.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.010885.
LITERATURE CITED
- Anderson JV, Horvath DP. Random sequencing of cDNAs and identification of mRNAs. Weed Sci. 2001;49:590–597. [Google Scholar]
- Aubert D, Chevillard M, Dorne AM, Arlaud G, Herzog M. Expression patterns of GASA genes in Arabidopsis thaliana: The GASA4 gene is up-regulated by gibberellins in meristematic regions. Plant Mol Biol. 1998;36:871–883. doi: 10.1023/a:1005938624418. [DOI] [PubMed] [Google Scholar]
- Beveridge CA, Symons GM, Turnbull CGN. Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol. 2000;123:689–698. doi: 10.1104/pp.123.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Chao WS, Anderson JV, Horvath DP. Sugar plays a role in inhibition of underground adventitious bud growth in leafy spurge (Euphorbia esula L.) Plant Physiol Suppl. 2000;124:46. [Google Scholar]
- Chao WS, Horvath DP, Anderson JV. Regulation of underground adventitious bud growth in leafy spurge (Euphorbia esula) Plant Physiol Suppl. 2001;308:79. [Google Scholar]
- Chaplin MF. Monosaccharides. In: Chaplin MF, Kennedy JF, editors. Carbohydrate Analysis: A Practical Approach. Ed 2. New York: IRL Press at Oxford University Press; 1994. pp. 1–10. [Google Scholar]
- Chen HH, Li PH, Brenner ML. Involvement of abscisic acid in potato cold acclimation. Plant Physiol. 1983;71:362–365. doi: 10.1104/pp.71.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline MG. Apical dominance. Bot Rev. 1991;57:318–358. [Google Scholar]
- Debeaujon I, Koornneef M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000;122:415–424. doi: 10.1104/pp.122.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devitt ML, Stafstrom JP. Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol Biol. 1995;29:255–265. doi: 10.1007/BF00043650. [DOI] [PubMed] [Google Scholar]
- Fobert PR, Coen ES, Murphy GJP, Doonan JH. Patterns of cell division revealed by transcriptional regulation of genes during the cell cycle in plants. EMBO J. 1994;13:616–624. doi: 10.1002/j.1460-2075.1994.tb06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin V, Lunness PA, Fobert PR, Towers M, Riou-Khamlichi C, Murray JAH, Coen E, Doonan JH. The expression of D-cyclin genes defines distinct developmental zones in snapdragon apical meristems and is locally regulated by the cycloidea gene. Plant Physiol. 2000;122:1137–1148. doi: 10.1104/pp.122.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau E, Hofte H, Orbovic V, Traas J. Gibberellin and ethylene control endoreduplication levels in the Arabidopsis thaliana hypocotyl. Planta. 1999;209:513–516. doi: 10.1007/PL00008123. [DOI] [PubMed] [Google Scholar]
- Gocal GFW, Pharis RP, Yeung EC, Pearce D. Changes after decapitation of indol-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv Tender Green. Plant Physiol. 1991;95:344–350. doi: 10.1104/pp.95.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S-L, Chao Y-C, Tong W-F, Yu S-M. Sugar coordinately and differentially regulates growth- and stress-related gene expression via a complex signal transduction network and multiple control mechanisms. Plant Physiol. 2001;125:877–890. doi: 10.1104/pp.125.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath DP. The role of specific plant organs and polar auxin transport in correlative inhibition of leafy spurge (Euphorbia esula) root buds. Can J Bot. 1998;76:1227–1231. [Google Scholar]
- Horvath DP. Role of mature leaves in inhibition of root bud growth in Euphorbia esula. Weed Sci. 1999;47:544–550. [Google Scholar]
- Horvath DP, Anderson JV. The effect of photosynthesis on underground adventitious shoot bud dormancy/quiescence in leafy spurge (Euphorbia esula L.) In: Viemont J-D, Crabbe JJ, editors. Second International Symposium on Plant Dormancy: Short Communications. Wallingford, UK: Presses de L'Universite d'Angers and CAB International; 2000. pp. 30–34. [Google Scholar]
- Huijser C, Kortstee A, Pego J, Weisbeek P, Wisman E, Smeekens S. The Arabidopsis SUCROSE UNCOUPLED-6 gene is identical to ABSCISIC ACID INSENSITIVE-4: involvement of abscisic acid in sugar responses. Plant J. 2000;23:577–585. doi: 10.1046/j.1365-313x.2000.00822.x. [DOI] [PubMed] [Google Scholar]
- Jang J-C, Leó P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laby RJ, Kincaid MS, Kim D, Gibson SI. The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J. 2000;23:587–596. doi: 10.1046/j.1365-313x.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- Perata P, Matsukura C, Vernieri P, Yamaguchi J. Sugar repression of a gibberellin-dependant signaling pathway in barley embryos. Plant Cell. 1997;9:2197–2208. doi: 10.1105/tpc.9.12.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde A, Howe GT, Olsen JE, Moritz T, Van Montegu M, Junttila O, Boerjan W. Molecular aspects of bud dormancy in trees. In: Jain SM, Minocha SC, editors. Molecular Biology of Woody Plants. Vol. 1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 89–134. [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M. The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast grr1p. Genes Dev. 1998;12:198–207. doi: 10.1101/gad.12.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. Ed 2. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sauter M, Mekhedov SL, Kende H. Gibberellin promotes histone H1 kinase activity and the expression of cdc2 and cyclin genes during the induction of rapid growth in deepwater rice internodes. Plant J. 1995;7:623–632. doi: 10.1046/j.1365-313x.1995.7040623.x. [DOI] [PubMed] [Google Scholar]
- Shi L, Olszewski NE. Gibberellin and abscisic acid regulate GAST1 expression at the level of transcription. Plant Mol Biol. 1998;38:1053–1060. doi: 10.1023/a:1006007315718. [DOI] [PubMed] [Google Scholar]
- Soni R, Carmichael JP, Shah ZH, Murray JA. A family of cyclin D homologues from plants differentially controlled by growth regulators and containing the conserved retinoblastoma protein interaction motif. Plant Cell. 1995;7:85–103. doi: 10.1105/tpc.7.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom JP, Ripley BD, Devitt ML, Drake B. Dormancy-associated gene expression in pea axillary buds: cloning and expression of PsDRM1 and PsDRM2. Planta. 1998;205:547–552. doi: 10.1007/s004250050354. [DOI] [PubMed] [Google Scholar]
- Vantard M, Cowling R, Delichere C. Cell cycle regulation of the microtubular cytoskeleton. Plant Mol Biol. 2000;43:691–703. doi: 10.1023/a:1006346107807. [DOI] [PubMed] [Google Scholar]
- Wang H, Qi Q, Schorr P, Cutler AJ, Crosby WL, Fowke LC. ICK1, a cyclin-dependent protein kinase inhibitor from Arabidopsis thaliana interacts with both Cdc2a and CycD3, and its expression is induced by abscisic acid. Plant J. 1998;15:501–510. doi: 10.1046/j.1365-313x.1998.00231.x. [DOI] [PubMed] [Google Scholar]
- White CN, Rivin CJ. Gibberellins and seed development in maize: II. Gibberellin synthesis inhibition enhances abscisic acid signaling in cultured embryos. Plant Physiol. 2000;122:1089–1098. doi: 10.1104/pp.122.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, van Lammeren AAM, Vermeer E, Vreugdenhil D. The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol. 1998;117:575–584. doi: 10.1104/pp.117.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]