Abstract
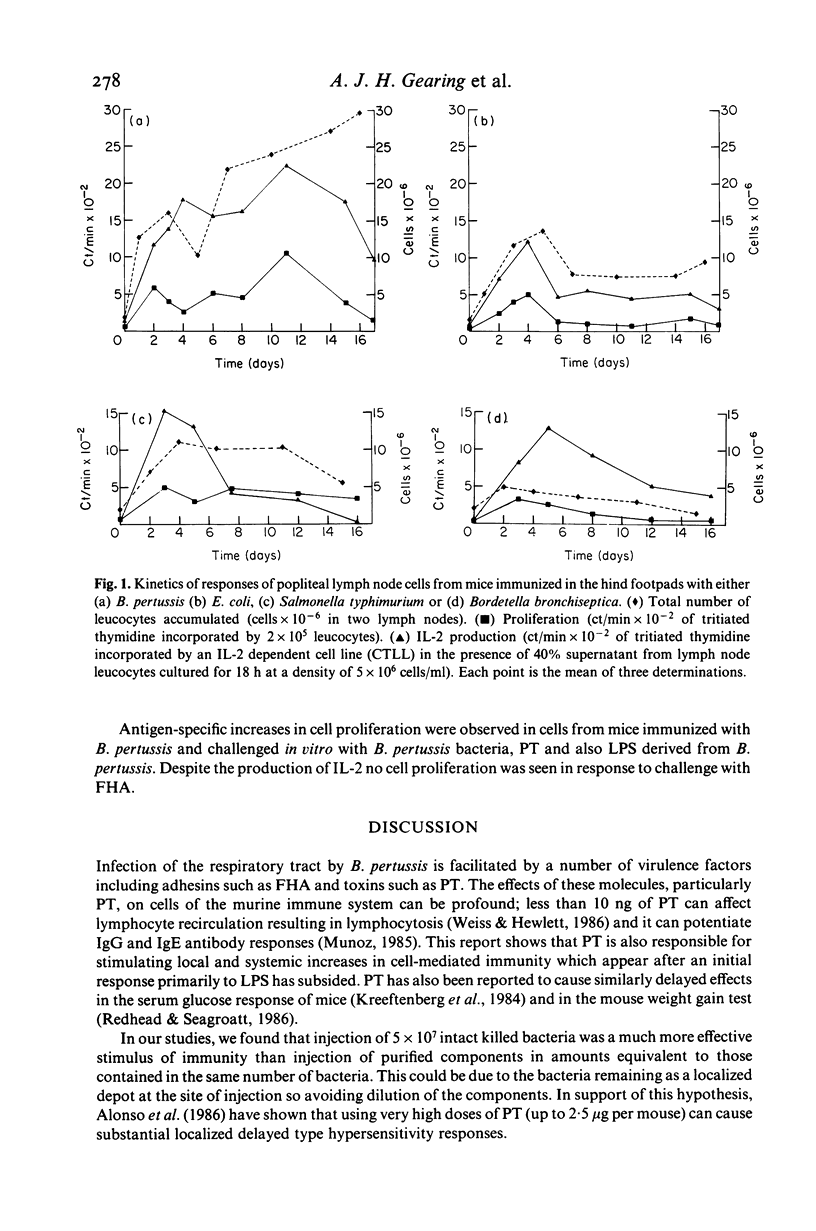
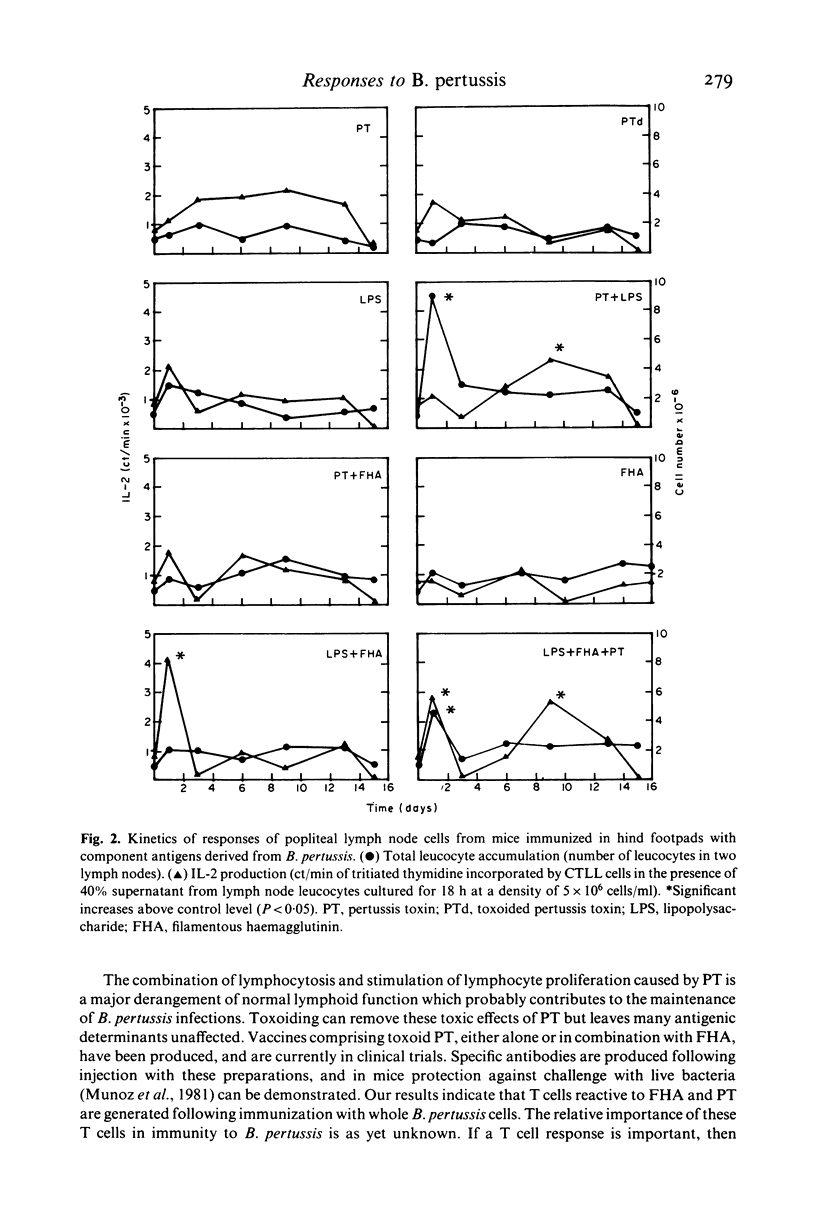
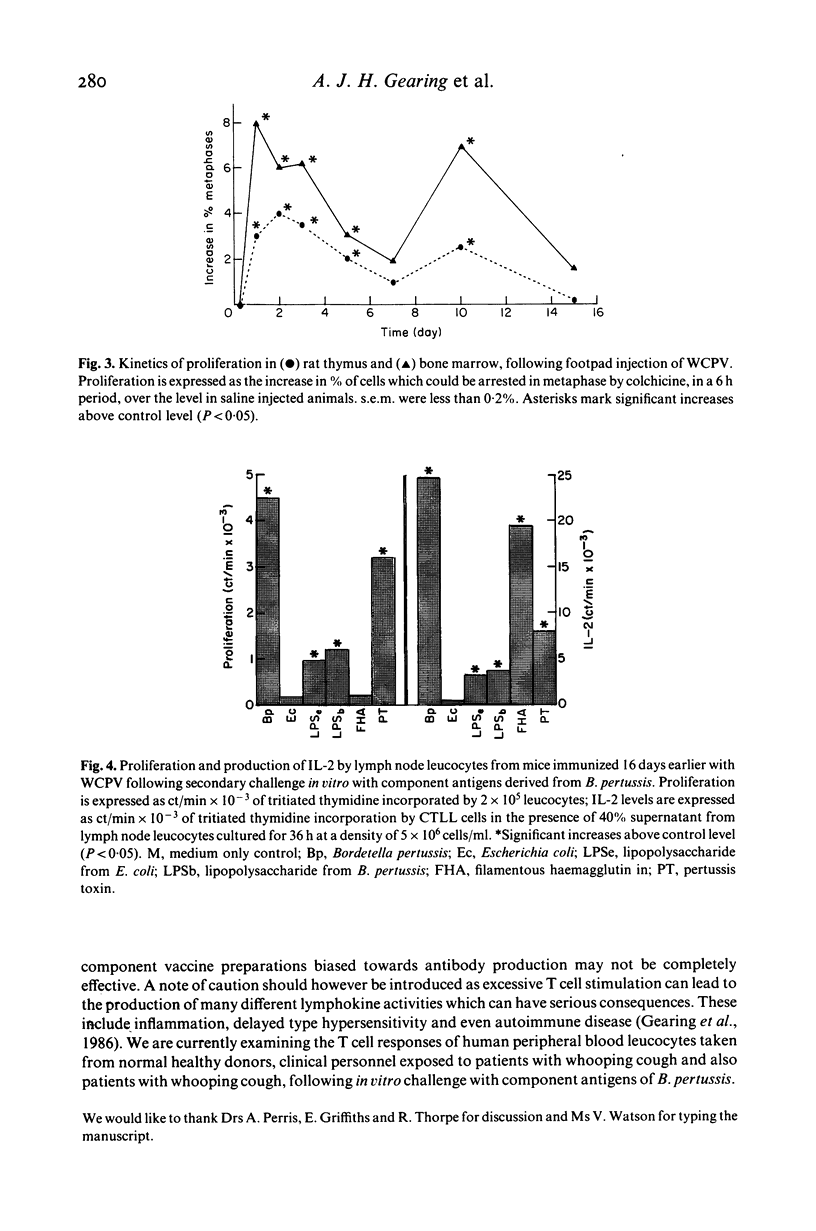
The cellular immune responses of Balb/c mice and Wistar rats immunized in hind footpads with intact killed Bordetella pertussis were found to differ from those of similar animals immunized with other bacteria including Bordetella bronchiseptica, Salmonella typhimurium and Escherichia coli. All the bacteria stimulated increases in cell number, proliferation and interleukin 2 (IL-2) production in popliteal lymph nodes which peaked 3-5 days after injection and decreased to resting levels by day 7. However, B. pertussis also caused a second peak in all three parameters at 11 days after immunization. This peak was not seen following injection with any of the other bacteria. Bordetella pertussis also caused systemic effects, increased cellular proliferation in bone marrow and thymus, with similar biphasic kinetics. It possesses a potent toxin, distinguishing it from the closely related B. bronchiseptica. The use of purified materials confirmed that the presence of this pertussis toxin (PT) was responsible for the later peak in stimulation, whereas lipopolysaccharide (LPS) in combination with PT and also the filamentous haemagglutinin (FHA) could mimic the early peak of stimulation. Primary immunization with B. pertussis was also shown to generate lymph node cells which responded in vitro to secondary challenge with B. pertussis cells, FHA or PT. Both proliferation and IL-2 production were enhanced, except with FHA which only increased IL-2 production. Lymph node cells from mice immunized with E. coli showed no such responses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth L. A., Robinson A., Irons L. I., Morgan C. P., Isaacs D. Antigens in whooping cough vaccine and antibody levels induced by vaccination of children. Lancet. 1983 Oct 15;2(8355):878–881. doi: 10.1016/s0140-6736(83)90869-3. [DOI] [PubMed] [Google Scholar]
- Edwards D. J., Rimmer J. J., Atkinson M. J., Perris A. D. Changes in plasma levels of calcium and in bone marrow mitosis after antigenic challenge in rats and mice. J Endocrinol. 1981 Sep;90(3):445–452. doi: 10.1677/joe.0.0900445. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Bird C. R., Callus M., Thorpe R. The effect of primary immunization and concanavalin A on the production of monoclonal natural antibodies. Hybridoma. 1986 Fall;5(3):243–247. doi: 10.1089/hyb.1986.5.243. [DOI] [PubMed] [Google Scholar]
- Gearing A. J., Johnstone A. P., Thorpe R. Production and assay of the interleukins. J Immunol Methods. 1985 Oct 24;83(1):1–27. doi: 10.1016/0022-1759(85)90053-5. [DOI] [PubMed] [Google Scholar]
- Irons L. I., MacLennan A. P. Isolation of the lymphocytosis promoting factor-haemagglutinin of Bordetella pertussis by affinity chromatography. Biochim Biophys Acta. 1979 Sep 29;580(1):175–185. doi: 10.1016/0005-2795(79)90208-3. [DOI] [PubMed] [Google Scholar]
- Kendrick P. L., Eldering G., Dixon M. K., Misner J. Mouse Protection Tests in the Study of Pertussis Vaccine: A Comparative Series Using the Intracerebral Route for Challenge. Am J Public Health Nations Health. 1947 Jul;37(7):803–810. [PMC free article] [PubMed] [Google Scholar]
- Kreeftenberg J. G., van Straaten-van de Kappelle I., de Wildt D. J., Terligen J. B., Peters W. J., Walvoort H. C. A biphasic serum glucose response in mice to inoculation with pertussis vaccine. J Biol Stand. 1984;12(2):151–157. doi: 10.1016/s0092-1157(84)80048-7. [DOI] [PubMed] [Google Scholar]
- Munoz J. J., Arai H., Cole R. L. Mouse-protecting and histamine-sensitizing activities of pertussigen and fimbrial hemagglutinin from Bordetella pertussis. Infect Immun. 1981 Apr;32(1):243–250. doi: 10.1128/iai.32.1.243-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman M. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev Infect Dis. 1979 May-Jun;1(3):401–412. doi: 10.1093/clinids/1.3.401. [DOI] [PubMed] [Google Scholar]
- Redhead K., Seagroatt V. The effects of purified components of Bordetella pertussis in the weight gain test for the toxicity testing of pertussis vaccines. J Biol Stand. 1986 Jan;14(1):57–65. doi: 10.1016/s0092-1157(86)80009-9. [DOI] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Aerosol infection of mice with Bordetella pertussis. Infect Immun. 1980 Jul;29(1):261–266. doi: 10.1128/iai.29.1.261-266.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Izumiya K., Sato H., Cowell J. L., Manclark C. R. Role of antibody to leukocytosis-promoting factor hemagglutinin and to filamentous hemagglutinin in immunity to pertussis. Infect Immun. 1981 Mar;31(3):1223–1231. doi: 10.1128/iai.31.3.1223-1231.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Spitz M., Gearing A., Callus M., Spitz L., Thorpe R. Interleukin-2 in vivo: production of and response to interleukin-2 in lymphoid organs undergoing a primary immune response to heterologous erythrocytes. Immunology. 1985 Mar;54(3):527–532. [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]


