Abstract
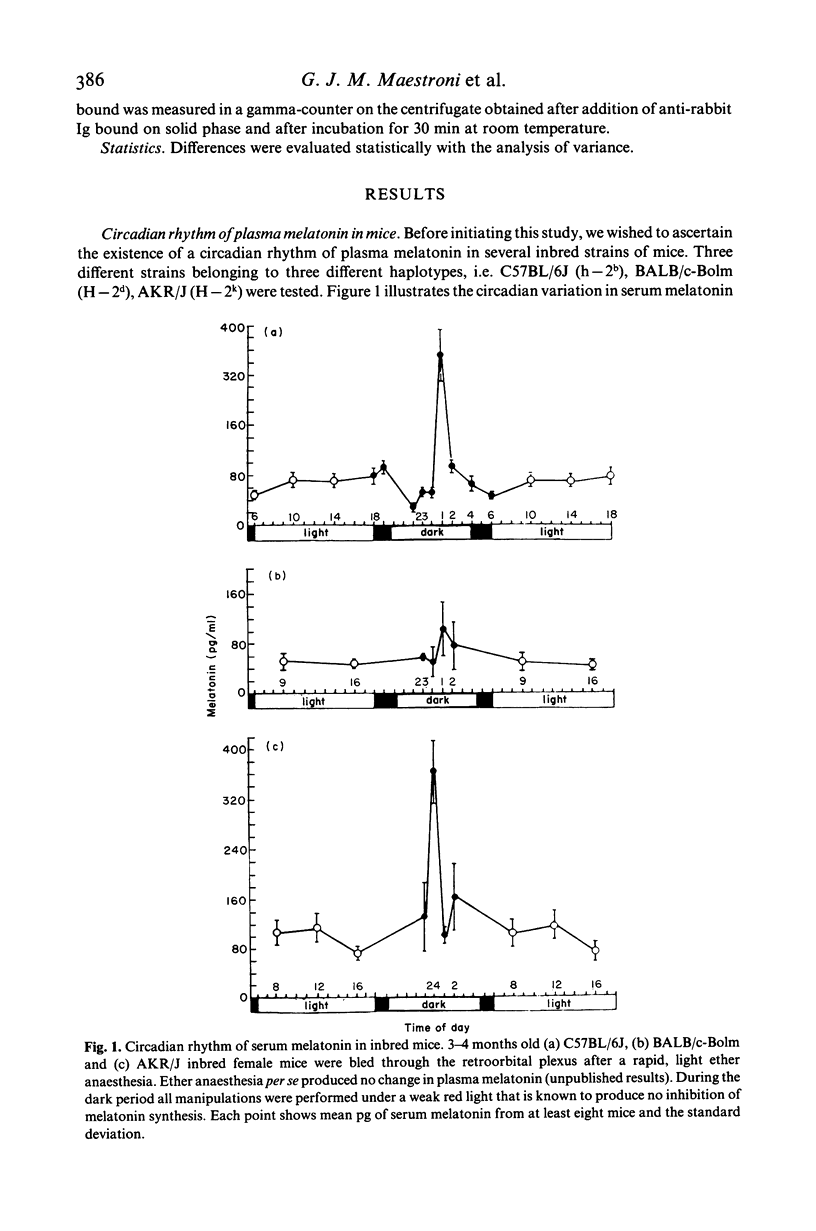
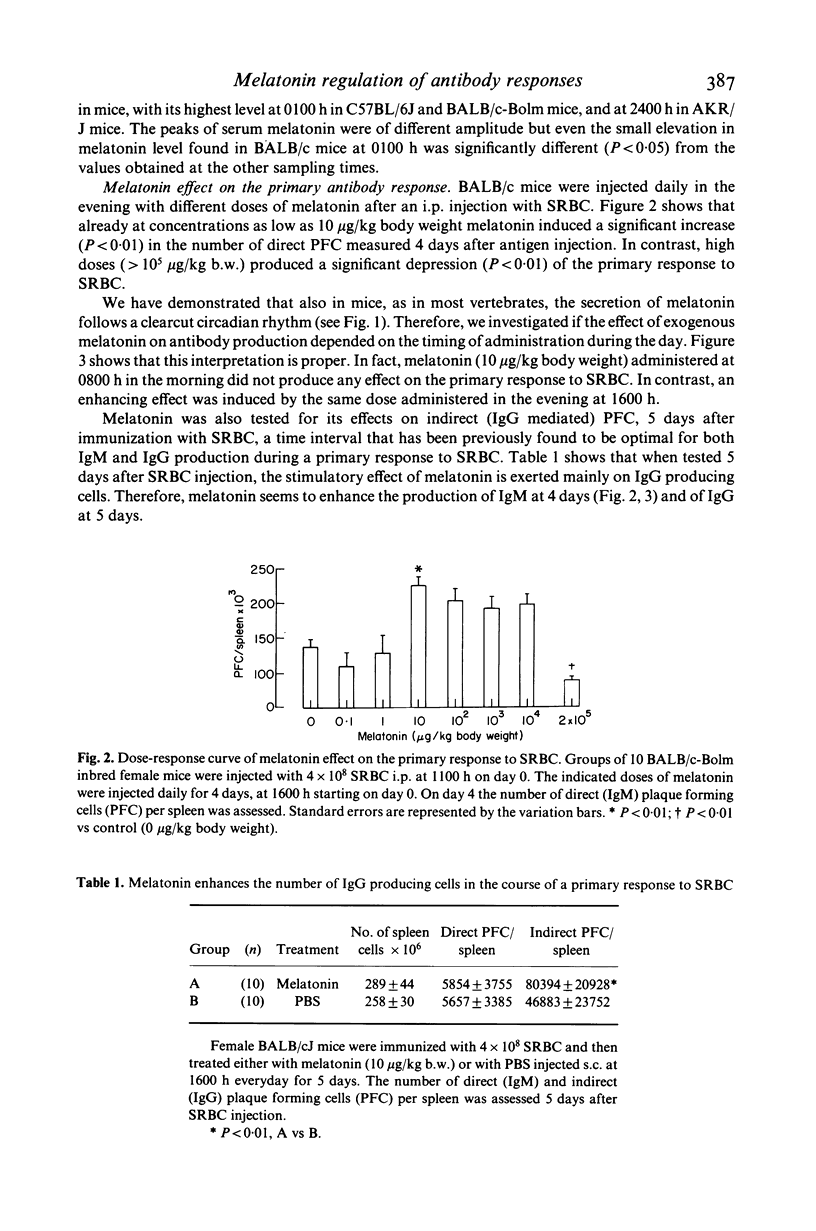
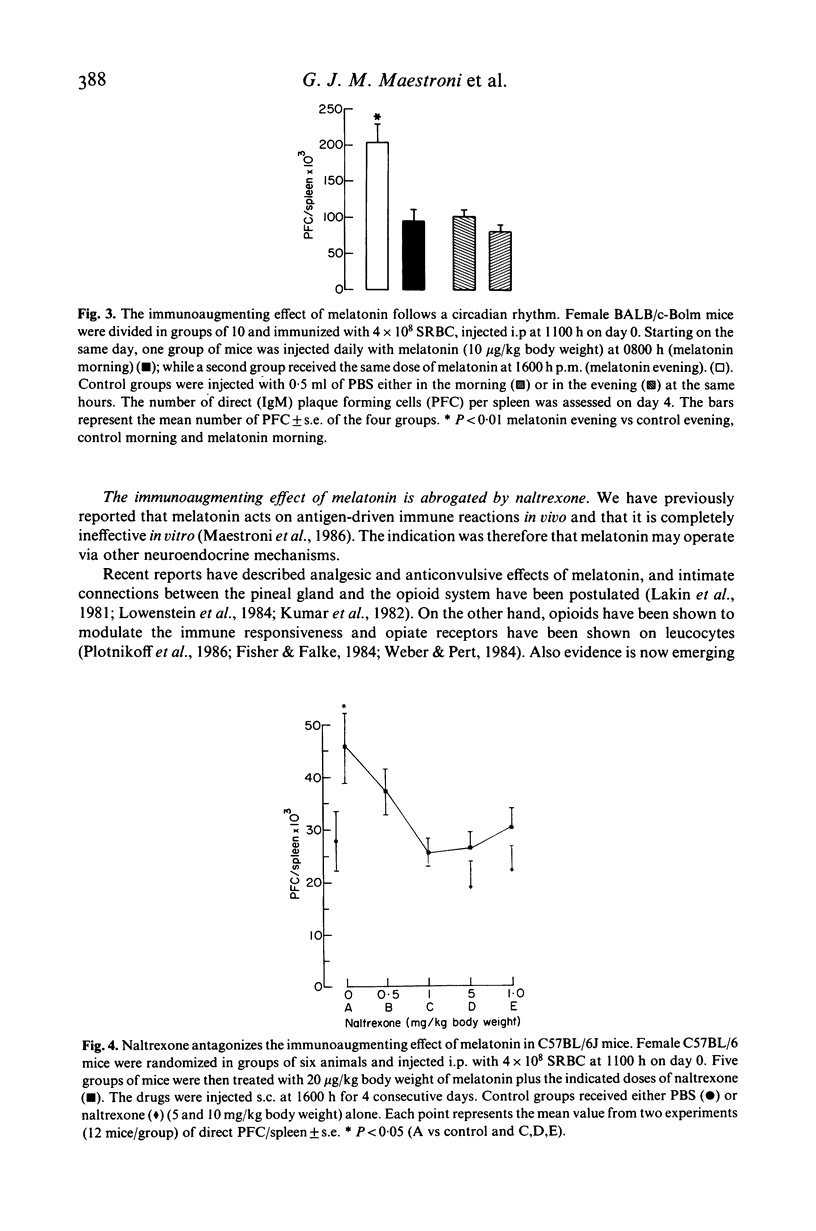
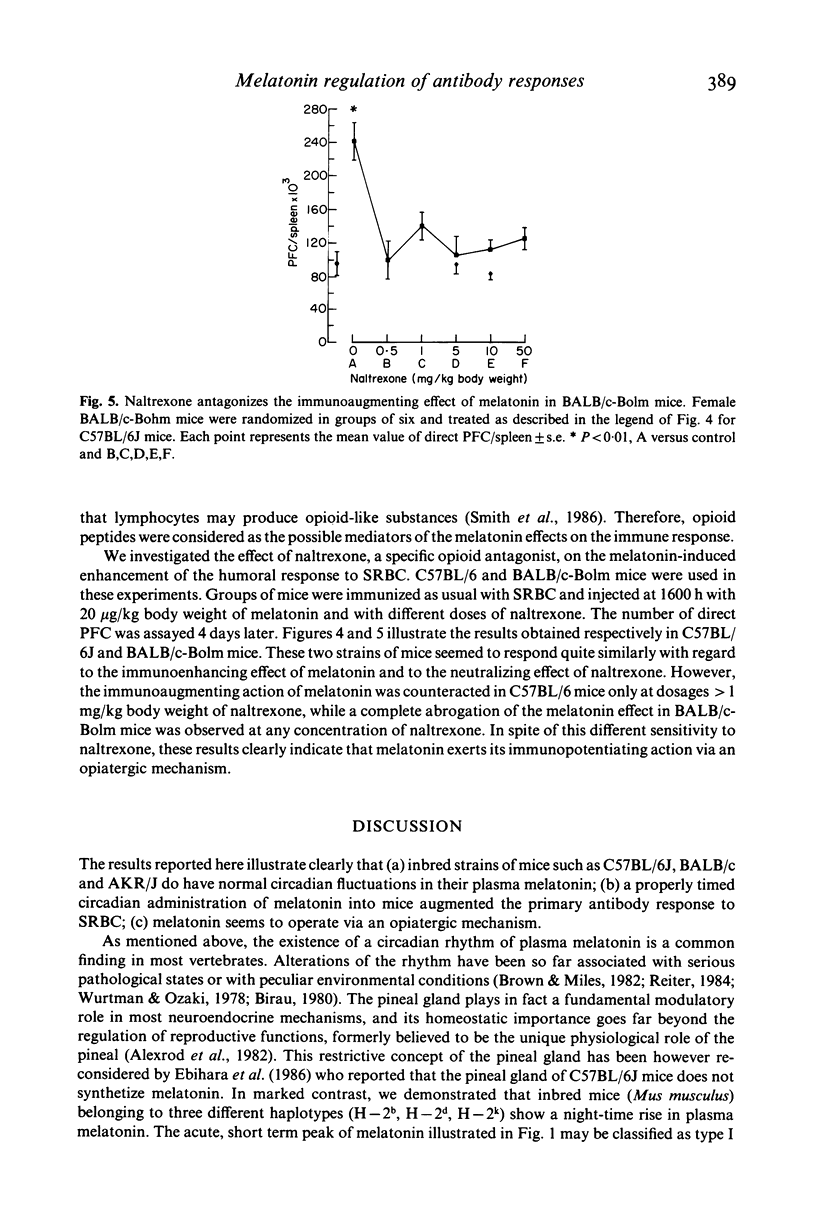
The pineal gland constitutes a major neuroendocrine organ in the brain. It transduces exogenous signals such as circadian and seasonal variations of light and temperature into proper hormonal changes which adjust and adapt internal endocrine functions. These pineal activities seem to be exerted via circadian synthesis and release of the indoleamine melatonin, a neurohormone secreted by the pineal itself. Alteration of circadian rhythms have been associated with affective disorders, psychosomatic diseases, cancer and many other pathologies. We have reported that functional and pharmacologic inhibition of melatonin synthesis results in depressed immune functions in vivo and that exogenous, evening administration of melatonin enhances antibody formation via an antigen-activated process and also antagonizes the immunosuppressive effects of corticosterone. We communicate here findings demonstrating that (a) three different inbred strains of mice possess a clear-cut cycle of melatonin levels in serum, (b) that melatonin administered in the evening enhances primary antibody response (IgM and IgG immunoglobulins) in vivo according to a dose-response behaviour and that (c) the opioid receptors blocker naltrexone antagonizes the immunostimulatory effect of melatonin. These findings point to a fundamental immunoregulatory role of circadian melatonin and to an activity of the neurohormone via opioid peptides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blalock J. E. The immune system as a sensory organ. J Immunol. 1984 Mar;132(3):1067–1070. [PubMed] [Google Scholar]
- Deguchi T., Axelrod J. Supersensitivity and subsensitivity of the beta-adrenergic receptor in pineal gland regulated by catecholamine transmitter. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2411–2414. doi: 10.1073/pnas.70.8.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara S., Marks T., Hudson D. J., Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986 Jan 31;231(4737):491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- Fischer E. G., Falke N. E. Beta-endorphin modulates immune functions. A review. Psychother Psychosom. 1984;42(1-4):195–204. doi: 10.1159/000287845. [DOI] [PubMed] [Google Scholar]
- Kumar M. S., Chen C. L., Sharp D. C., Liu J. M., Kalra P. S., Kalra S. P. Diurnal fluctuations in methionine-enkephalin levels in the hypothalamus and preoptic area of the male rat: effects of pinealectomy. Neuroendocrinology. 1982;35(1):28–31. doi: 10.1159/000123350. [DOI] [PubMed] [Google Scholar]
- Lakin M. L., Miller C. H., Stott M. L., Winters W. D. Involvement of the pineal gland and melatonin in murine analgesia. Life Sci. 1981 Dec 14;29(24):2543–2551. doi: 10.1016/0024-3205(81)90710-4. [DOI] [PubMed] [Google Scholar]
- Lowenstein P. R., Pereyra E. N., González Solveyra C., Cardinali D. P. Effect of naloxone on the nocturnal rise of rat pineal melatonin content. Eur J Pharmacol. 1984 Feb 17;98(2):261–264. doi: 10.1016/0014-2999(84)90598-3. [DOI] [PubMed] [Google Scholar]
- Maestroni G. J., Conti A., Pierpaoli W. Role of the pineal gland in immunity. Circadian synthesis and release of melatonin modulates the antibody response and antagonizes the immunosuppressive effect of corticosterone. J Neuroimmunol. 1986 Nov;13(1):19–30. doi: 10.1016/0165-5728(86)90047-0. [DOI] [PubMed] [Google Scholar]
- Pierpaoli W., Maestroni G. J. Pharmacologic control of the hormonally modulated immune response. III. Prolongation of allogeneic skin graft rejection and prevention of runt disease by a combination of drugs acting on neuroendocrine functions. J Immunol. 1978 May;120(5):1600–1603. [PubMed] [Google Scholar]
- Pierpaoli W., Maestroni G. J. Pharmacological control of the hormonally modulated immune response. II. Blockade of antibody production by a combination of drugs acting on neuroendocrine functions. Its prevention by gonadotropins and corticotrophin. Immunology. 1978 Mar;34(3):419–430. [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W., Maestroni G. J. Pharmacological control of the immune response by blockade of the early hormonal changes following antigen injection. Cell Immunol. 1977 Jun 15;31(2):355–363. doi: 10.1016/0008-8749(77)90037-5. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Morrill A. C., Meyer W. J., 3rd, Blalock J. E. Corticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. 1986 Jun 26-Jul 2Nature. 321(6073):881–882. doi: 10.1038/321881a0. [DOI] [PubMed] [Google Scholar]
- Waterfield A. A., Lord J. A., Hughes J., Kosterlitz H. W. Differences in the inhibitory effects of normorphine and opioid peptides on the responses of the vasa deferentia of two strains of mice. Eur J Pharmacol. 1978 Jan 15;47(2):249–250. doi: 10.1016/0014-2999(78)90399-0. [DOI] [PubMed] [Google Scholar]


