Abstract
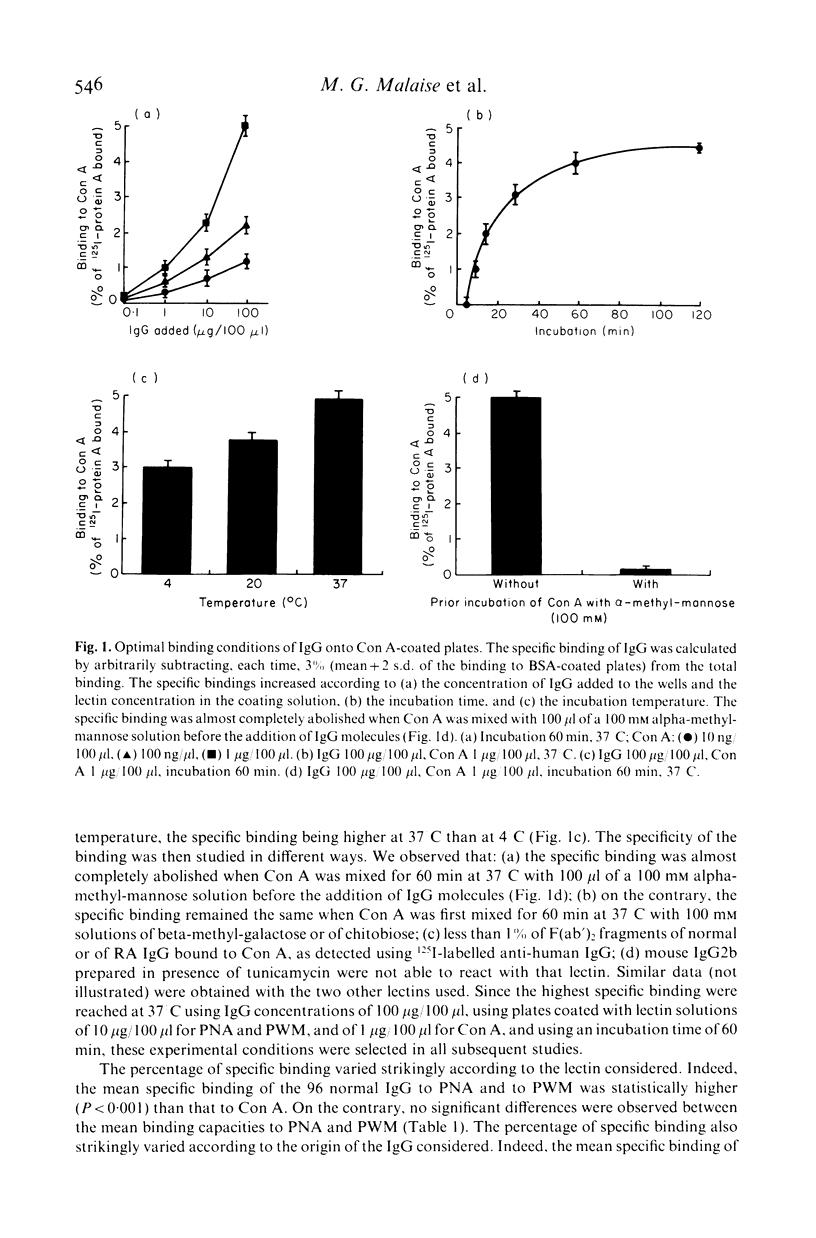
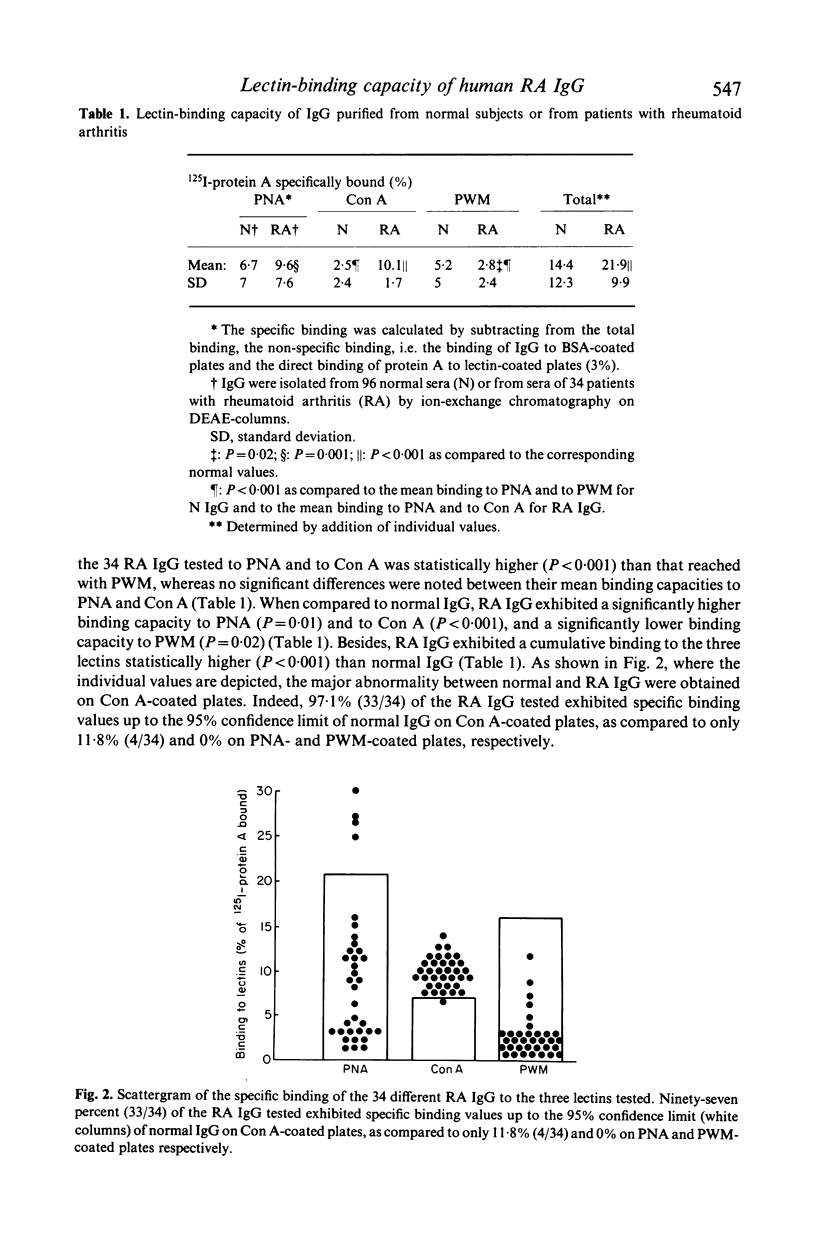
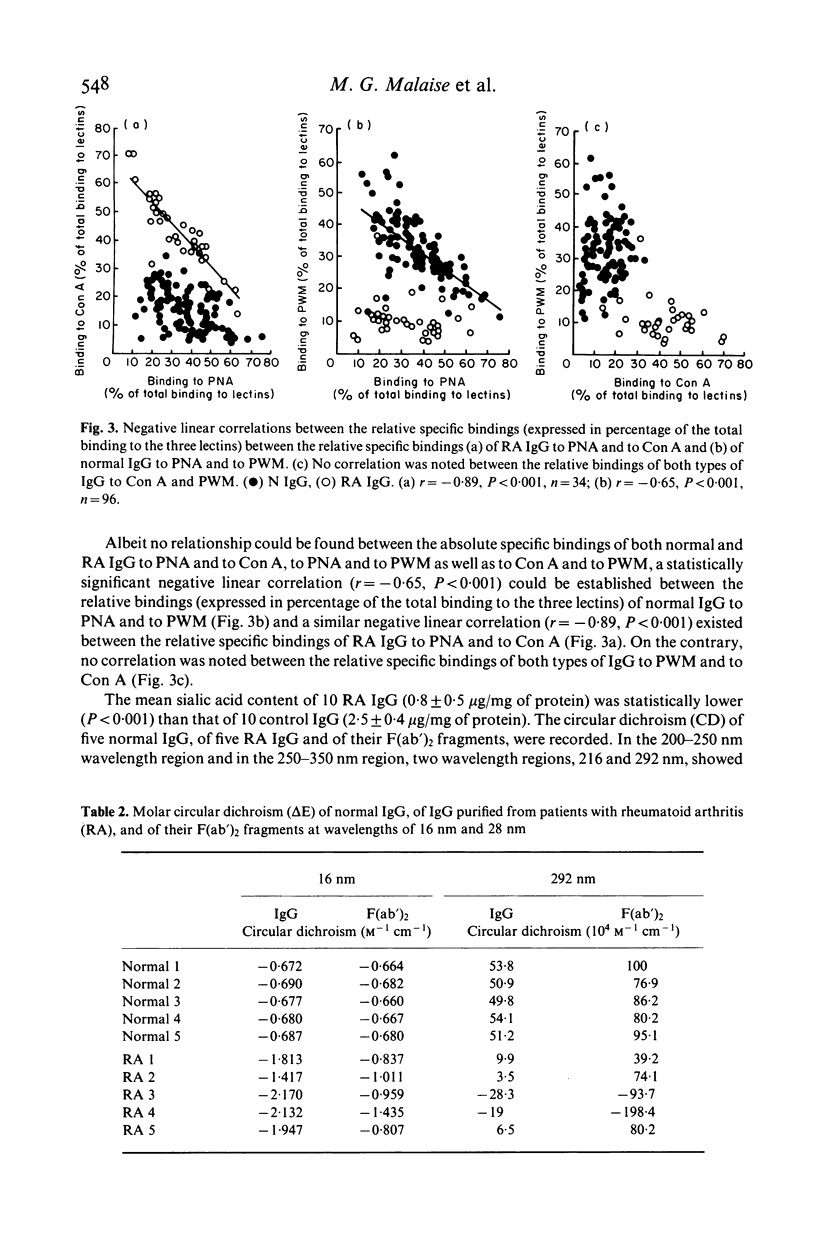
A solid phase radioimmunoassay was set up for direct measurement of the binding capacity of human IgG to three lectins recognizing different carbohydrates of the Fc domain, i.e. peanut agglutinin (PNA), Concanavalin A (Con A) and pokeweed mitogen (PWM) which mainly bind to beta-galactose, alpha-mannose and dimers of N-acetyl-beta-glucosamine respectively. The mean specific binding of the 96 normal IgG tested to PNA and to PWM was statistically higher (P less than 0.001) than that to Con A, whereas no significant differences were observed between the mean specific bindings to PNA and to PWM. A statistically significant linear negative correlation could be established only between the relative bindings (expressed in percentage of the total binding to the three lectins) to PNA and to PWM (r = -0.65, P less than 0.001). The mean specific binding of IgG purified from 34 patients suffering from rheumatoid arthritis (RA) to PNA and to Con A was statistically higher (P less than 0.001) than that reached with PWM, whereas no significant differences were noted between their mean binding capacities to PNA and to Con A. When compared to normal IgG, only four out of 34 RA IgG exhibited a significantly higher binding capacity to PNA, whereas all but one RA IgG possessed a significantly higher binding capacity to Con A. Accordingly, the mean specific binding of RA IgG to Con A was significantly higher than that of normal IgG (P less than 0.001). Besides (and contrary to normal IgG), a statistically significant negative linear correlation was noted between the relative bindings of RA IgG to PNA and to Con A (r = -0.89, P less than 0.001). All the five RA IgG tested exhibited an abnormal circular dichroism. Our data suggest that, by altered steric conformation and glycosylation, mannosyl-residues of RA IgG become prominent or terminal or both, and are therefore able to react more effectively with Con A than normal IgG do.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dodon M. D., Quash G. A. The antigenicity of asialylated IgG: its relationship to rheumatoid factor. Immunology. 1981 Mar;42(3):401–408. [PMC free article] [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Huber R., Deisenhofer J., Colman P. M., Matsushima M., Palm W. Crystallographic structure studies of an IgG molecule and an Fc fragment. Nature. 1976 Dec 2;264(5585):415–420. doi: 10.1038/264415a0. [DOI] [PubMed] [Google Scholar]
- Johnson P. M., Watkins J., Scopes P. M., Tracey B. M. Differences in serum IgG structure in health and rheumatoid disease. Circular dichroism studies. Ann Rheum Dis. 1974 Jul;33(4):366–370. doi: 10.1136/ard.33.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinet J. P., Hunt J., Foidart J. B., Desoroux A., Mahieu P. Ex vivo perfusion of plasma over protein A columns in human mammary adenocarcinoma. Evidence for a protein A leaking by radioimmunoassay. Eur J Clin Invest. 1986 Feb;16(1):43–49. doi: 10.1111/j.1365-2362.1986.tb01306.x. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Keller J., Baenziger J., Kornfeld S. The structure of the glycopeptide of human gamma G myeloma proteins. J Biol Chem. 1971 May 25;246(10):3259–3268. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lunec J., Blake D. R., McCleary S. J., Brailsford S., Bacon P. A. Self-perpetuating mechanisms of immunoglobulin G aggregation in rheumatoid inflammation. J Clin Invest. 1985 Dec;76(6):2084–2090. doi: 10.1172/JCI112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahieu P. M., Winand R. J. Carbohydrate and amino-acid composition of human glomerular-basement-membrane fractions purified by affinity chromatography. Eur J Biochem. 1973 Aug 1;37(1):157–163. doi: 10.1111/j.1432-1033.1973.tb02970.x. [DOI] [PubMed] [Google Scholar]
- Malaise M. G., Foidart J. B., Hauwaert C., Mahieu P., Franchimont P. In vivo studies on the mononuclear phagocyte system Fc receptor function in rheumatoid arthritis. Correlations with clinical and immunological variables. J Rheumatol. 1985 Feb;12(1):33–42. [PubMed] [Google Scholar]
- Malaise M. G., Franchimont P., Houssier C., Closset J., Hennen G., Mahieu P. R. In vitro studies on the Fc-receptor function of mononuclear phagocytes in rheumatoid arthritis: relation between the Fc-receptor blockade and the concanavalin A-binding capacity of autologous immunoglobulin G. J Clin Immunol. 1986 Nov;6(6):442–456. doi: 10.1007/BF00915250. [DOI] [PubMed] [Google Scholar]
- Malaise M. G., Hauwaert C., Franchimont P., Danneskiold-Samsoe B., Bach-Andersen R., Gross D., Gerber H., Gerschpacher H., Stocker H., Bolla K. Treatment of active rheumatoid arthritis with slow intravenous injections of thymopentin. A double-blind placebo-controlled randomised study. Lancet. 1985 Apr 13;1(8433):832–836. doi: 10.1016/s0140-6736(85)92205-6. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Mizuochi T., Taniguchi T., Shimizu A., Kobata A. Structural and numerical variations of the carbohydrate moiety of immunoglobulin G. J Immunol. 1982 Nov;129(5):2016–2020. [PubMed] [Google Scholar]
- Nose M., Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6632–6636. doi: 10.1073/pnas.80.21.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh R. B., Dwek R. A., Sutton B. J., Fernandes D. L., Leung A., Stanworth D., Rademacher T. W., Mizuochi T., Taniguchi T., Matsuta K. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985 Aug 1;316(6027):452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SOBER H. A., PETERSON E. A. Protein chromatography on ion exchange cellulose. Fed Proc. 1958 Dec;17(4):1116–1126. [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Occurrence of alpha-D-galactosyl residues in the thyroglobulins from several species. Localization in the saccharide chains of the complex carbohydrate units. J Biol Chem. 1984 Aug 10;259(15):9858–9866. [PubMed] [Google Scholar]
- WARREN L. Sialic acid in human semen and in the male genital tract. J Clin Invest. 1959 May;38(5):755–761. doi: 10.1172/JCI103856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Terao T., Osawa T. Carbohydrate-binding specificity of pokeweed mitogens. Biochim Biophys Acta. 1978 Jan 18;538(2):384–396. doi: 10.1016/0304-4165(78)90366-5. [DOI] [PubMed] [Google Scholar]


