Abstract
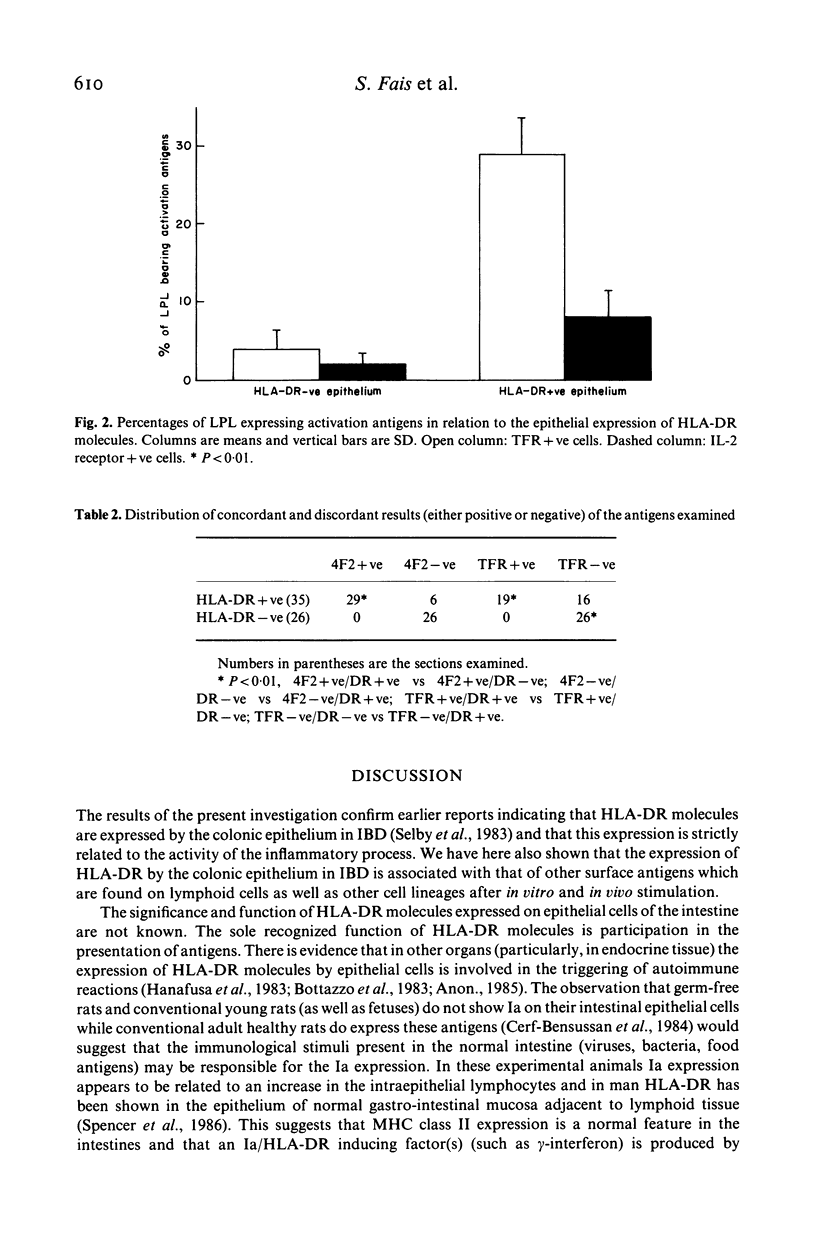
The colonic epithelial expression of HLA-DR molecules and of other markers of cell membrane perturbation was investigated by immunofluorescence in biopsy specimens from patients with ulcerative colitis and Crohn's disease. It was found that in virtually all specimens from either groups showing active inflammation there was a diffuse epithelial expression of HLA-DR molecules. There was no relation between the grading of active inflammation and the epithelial expression of HLA-DR antigens. The epithelium of virtually all specimens from the macro and microscopically uninvolved areas of patients with active colitis and from patients with histologically quiescent colitis showed no detectable expression of HLA-DR molecules. The counts of isolated lamina propria lymphocytes expressing the transferrin receptor and the interleukin 2 receptor were higher in specimens with HLA-DR+ epithelium than in those with a HLA-DR- epithelium. Twenty-nine of the 35 (83%) HLA-DR positive specimens proved to express the 4F2 antigen on their epithelium and 19 (54%) were positive for the transferrin receptor. All sections positive for either the 4F2 antigen or the transferrin receptor were also HLA-DR positive while all HLA-DR negative sections were also negative for either of the two other markers. Data in this study suggest that in active IBD the epithelial participation in active inflammation is associated with a sequence of cell membrane rearrangements, and that the expression of HLA-DR molecules is a part of this sequence.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Mason D. W. Induction of Ia antigen in rat epidermal cells and gut epithelium by immunological stimuli. J Exp Med. 1982 Dec 1;156(6):1665–1676. doi: 10.1084/jem.156.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzo G. F., Pujol-Borrell R., Hanafusa T., Feldmann M. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983 Nov 12;2(8359):1115–1119. doi: 10.1016/s0140-6736(83)90629-3. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Quaroni A., Kurnick J. T., Bhan A. K. Intraepithelial lymphocytes modulate Ia expression by intestinal epithelial cells. J Immunol. 1984 May;132(5):2244–2252. [PubMed] [Google Scholar]
- Corte G., Damiani G., Calabi F., Fabbi M., Bargellesi A. Analysis of HLA-DR polymorphism by two-dimensional peptide mapping. Proc Natl Acad Sci U S A. 1981 Jan;78(1):534–538. doi: 10.1073/pnas.78.1.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotner T., Williams J. M., Christenson L., Shapiro H. M., Strom T. B., Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med. 1983 Feb 1;157(2):461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy K., Van Heyningen V., Cohen B. B., Deane D. L., Steel C. M. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982 Nov;12(11):942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Mayrhofer G., Pugh C. W., Barclay A. N. The distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983 Feb;13(2):112–122. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- Mittler R. S., Rao P. E., Talle M. A., Look R., Goldstein G. Cell membrane perturbation of resting T cells and thymocytes causes display of activation antigens. J Exp Med. 1983 Jul 1;158(1):99–111. doi: 10.1084/jem.158.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natali P. G., De Martino C., Quaranta V., Nicotra M. R., Frezza F., Pellegrino M. A., Ferrone S. Expression of Ia-like antigens in normal human nonlymphoid tissues. Transplantation. 1981 Jan;31(1):75–78. doi: 10.1097/00007890-198101000-00017. [DOI] [PubMed] [Google Scholar]
- Scott H., Solheim B. G., Brandtzaeg P., Thorsby E. HLA-DR-like antigens in the epithelium of the human small intestine. Scand J Immunol. 1980;12(1):77–82. doi: 10.1111/j.1365-3083.1980.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Mason D. Y., Jewell D. P. Expression of HLA-DR antigens by colonic epithelium in inflammatory bowel disease. Clin Exp Immunol. 1983 Sep;53(3):614–618. [PMC free article] [PubMed] [Google Scholar]
- Shorter R. G., Spencer R. J., Hallenbeck G. A. Kinetic studies of the epithelial cells of the rectal mucosa in normal subjects and patients with ulcerative colitis. Gut. 1966 Dec;7(6):593–596. doi: 10.1136/gut.7.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J., Finn T., Isaacson P. G. Expression of HLA-DR antigens on epithelium associated with lymphoid tissue in the human gastrointestinal tract. Gut. 1986 Feb;27(2):153–157. doi: 10.1136/gut.27.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland R., Delia D., Schneider C., Newman R., Kemshead J., Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Malizia G., Oakes J., Losowsky M. S., Janossy G. Expression of the common acute lymphoblastic leukaemia antigen (CALLA gp100) in the brush border of normal jejunum and jejunum of patients with coeliac disease. J Clin Pathol. 1985 Sep;38(9):1002–1006. doi: 10.1136/jcp.38.9.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppi G., Gasparini R., Mantovanelli F., Gobio-Casali L., Astolfi R., Crovari P. Diet and antibody response to vaccinations in healthy infants. Lancet. 1983 Jul 2;2(8340):11–14. doi: 10.1016/s0140-6736(83)90004-1. [DOI] [PubMed] [Google Scholar]
- van Heyningen V., Guy K., Newman R., Steel C. M. Human MHC class II molecules as differentiation markers. Immunogenetics. 1982;16(5):459–469. doi: 10.1007/BF00372104. [DOI] [PubMed] [Google Scholar]