Abstract
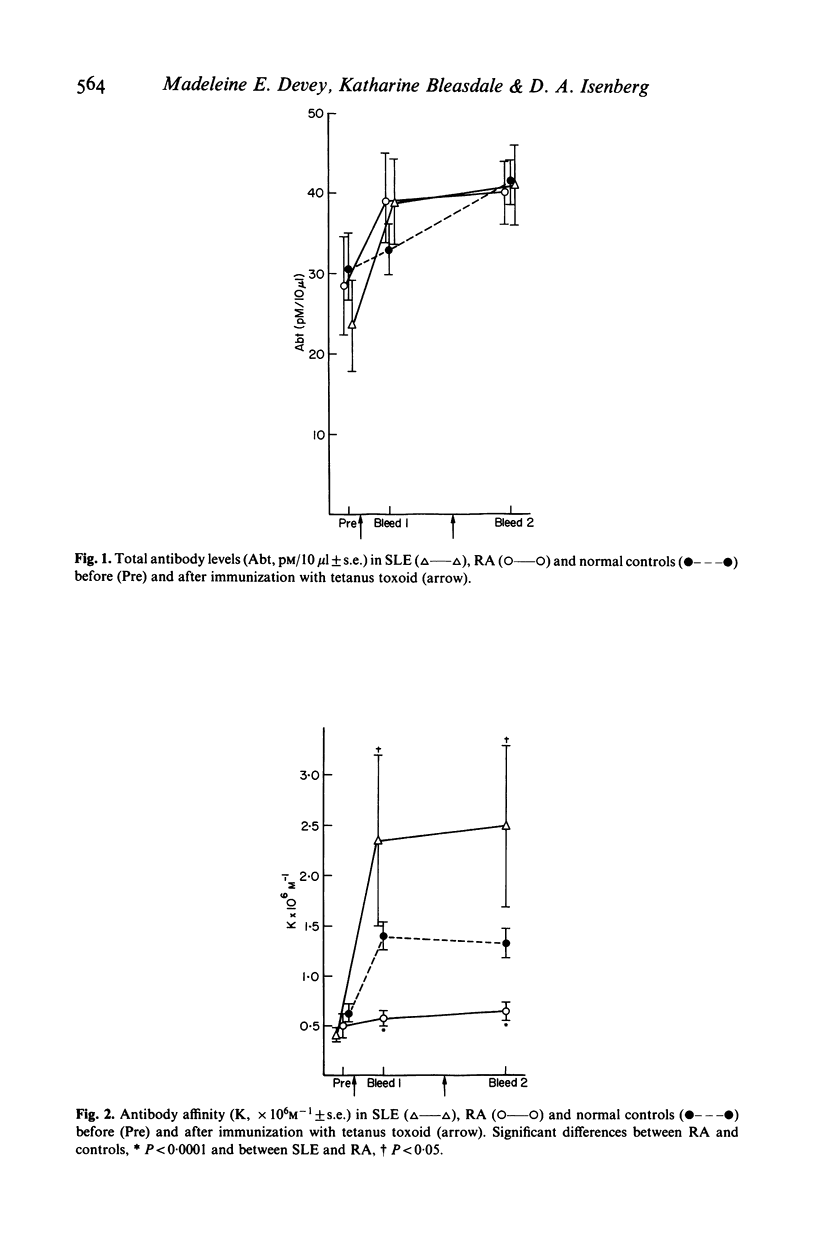
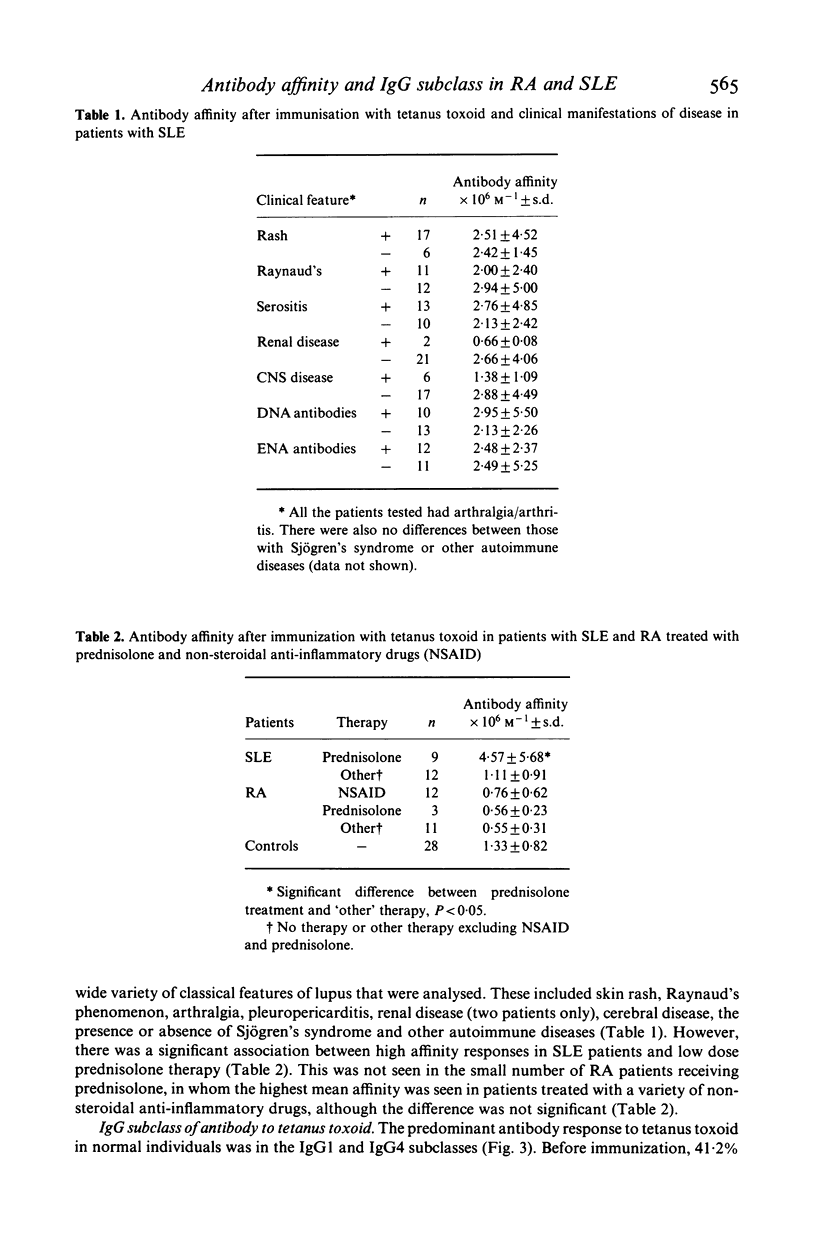
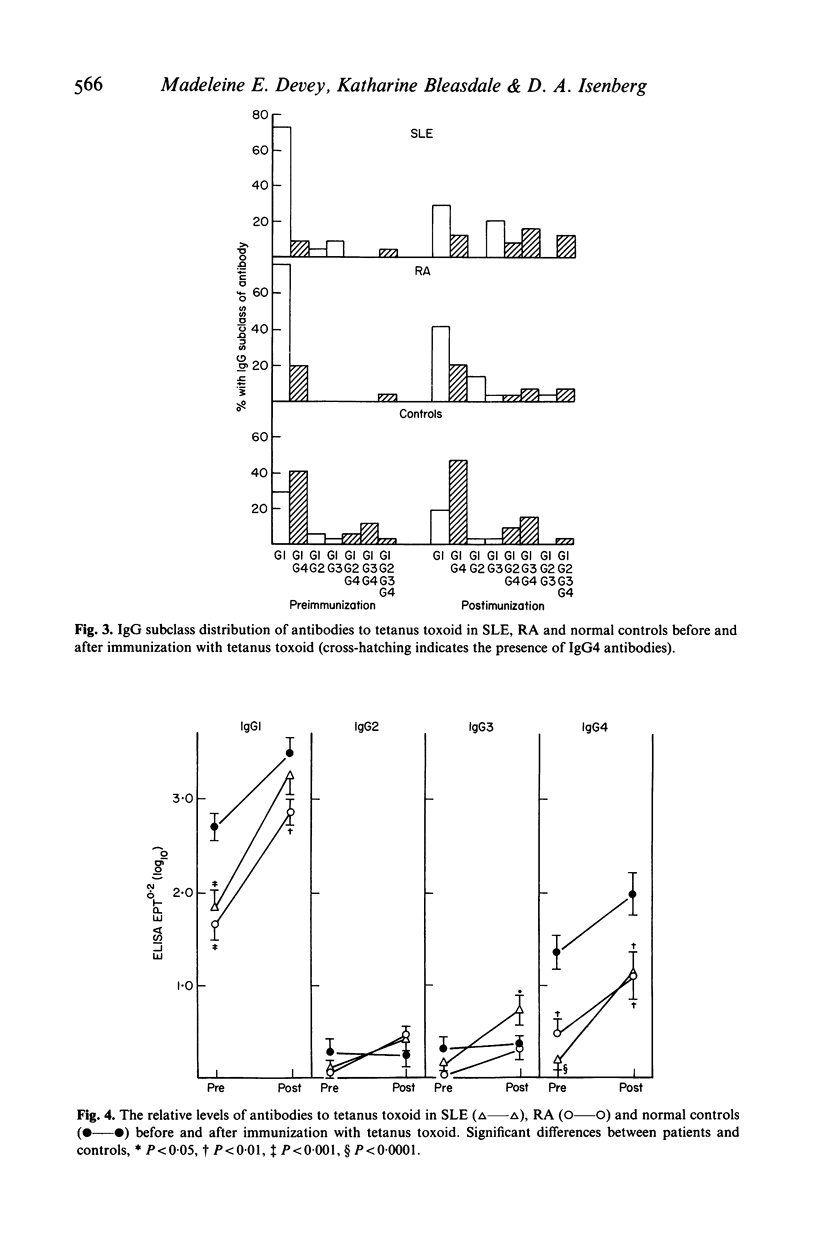
Significant differences in both the affinity and IgG subclass of antibodies produced after immunization with tetanus toxoid have been demonstrated in patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) compared to healthy controls. Patients with RA failed to show affinity maturation although they produced similar amounts of antibody to the controls. Some patients with SLE produced very high affinity antibodies although there was a wide spectrum of response. Antibodies to tetanus toxoid in controls were predominantly IgG1 and IgG4 but in RA and SLE there was either a restricted IgG1 response or a more general response in all the IgG subclasses. It is likely that these differences in response reflect the underlying disorders in immunoregulation present in patients with these diseases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. E., Howard C. R., Zuckerman A. J., Steward M. W. Affinity of antibody responses in man to hepatitis B vaccine determined with synthetic peptides. Lancet. 1984 Jul 28;2(8396):184–187. doi: 10.1016/s0140-6736(84)90479-3. [DOI] [PubMed] [Google Scholar]
- Devey M. E., Bleasdale K. M., French M. A., Harrison G. The IgG4 subclass is associated with a low affinity antibody response to tetanus toxoid in man. Immunology. 1985 Jul;55(3):565–567. [PMC free article] [PubMed] [Google Scholar]
- Devey M. E., Bleasdale K., Stanley C., Steward M. W. Failure of affinity maturation leads to increased susceptibility to immune complex glomerulonephritis. Immunology. 1984 Jun;52(2):377–383. [PMC free article] [PubMed] [Google Scholar]
- Hammarström L., Smith C. I. IgG subclass changes in response to vaccination. Monogr Allergy. 1986;19:241–252. [PubMed] [Google Scholar]
- Hirata F., Schiffmann E., Venkatasubramanian K., Salomon D., Axelrod J. A phospholipase A2 inhibitory protein in rabbit neutrophils induced by glucocorticoids. Proc Natl Acad Sci U S A. 1980 May;77(5):2533–2536. doi: 10.1073/pnas.77.5.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Posnett D. N., Kunkel H. G. Human malignant T cells capable of inducing an immunoglobulin class switch. J Exp Med. 1985 Jan 1;161(1):134–144. doi: 10.1084/jem.161.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi M., Kuritani T., Kubagawa H., Cooper M. D. IgG subclass expression by human B lymphocytes and plasma cells: B lymphocytes precommitted to IgG subclass can be preferentially induced by polyclonal mitogens with T cell help. J Immunol. 1983 Feb;130(2):671–677. [PubMed] [Google Scholar]
- Mitchell D. M., Fitzharris P., Knight R. A., Schild G. C., Snaith M. L. Kinetics of specific anti-influenza antibody production by cultured lymphocytes from patients with systemic lupus erythematosus following influenza immunization. Clin Exp Immunol. 1982 Aug;49(2):290–296. [PMC free article] [PubMed] [Google Scholar]
- Oxelius V. A. Immunoglobulin G (IgG) subclasses and human disease. Am J Med. 1984 Mar 30;76(3A):7–18. doi: 10.1016/0002-9343(84)90314-0. [DOI] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Sarvas H. O., Seppälä I. J., Tähtinen T., Péterfy F., Mäkelä O. Mouse IgG antibodies have subclass associated affinity differences. Mol Immunol. 1983 Mar;20(3):239–246. doi: 10.1016/0161-5890(83)90062-7. [DOI] [PubMed] [Google Scholar]
- Shakib F., Stanworth D. R. Human IgG subclasses in health and disease. (A review). Part II. Ric Clin Lab. 1980 Oct-Dec;10(4):561–580. doi: 10.1007/BF02906696. [DOI] [PubMed] [Google Scholar]
- Soothill J. F., Steward M. W. The immunopathological significance of the heterogeneity of antibody affinity. Clin Exp Immunol. 1971 Aug;9(2):193–199. [PMC free article] [PubMed] [Google Scholar]
- Steward M. W., Lew A. M. The importance of antibody affinity in the performance of immunoassays for antibody. J Immunol Methods. 1985 Apr 22;78(2):173–190. doi: 10.1016/0022-1759(85)90074-2. [DOI] [PubMed] [Google Scholar]
- Steward M. W., Petty R. E. The use of ammonium sulphate globulin precipitation for determination of affinity of anti-protein antibodies in mouse serum. Immunology. 1972 May;22(5):747–756. [PMC free article] [PubMed] [Google Scholar]
- Tojo T., Friou G. J., Spiegelberg H. L. Immunoglobulin G subclass of human antinuclear antibodies. Clin Exp Immunol. 1970 Jan;6(1):145–151. [PMC free article] [PubMed] [Google Scholar]
- Yu D. T., Peter J. B. Cellular immunological aspects of rheumatoid arthritis. Semin Arthritis Rheum. 1974 Fall;4(1):25–52. doi: 10.1016/0049-0172(74)90016-x. [DOI] [PubMed] [Google Scholar]


