Abstract
Evolution of resistance by pests is the main threat to long-term insect control by transgenic crops that produce Bacillus thuringiensis (Bt) toxins. Because inheritance of resistance to the Bt toxins in transgenic crops is typically recessive, DNA-based screening for resistance alleles in heterozygotes is potentially much more efficient than detection of resistant homozygotes with bioassays. Such screening, however, requires knowledge of the resistance alleles in field populations of pests that are associated with survival on Bt crops. Here we report that field populations of pink bollworm (Pectinophora gossypiella), a major cotton pest, harbored three mutant alleles of a cadherin-encoding gene linked with resistance to Bt toxin Cry1Ac and survival on transgenic Bt cotton. Each of the three resistance alleles has a deletion expected to eliminate at least eight amino acids upstream of the putative toxin-binding region of the cadherin protein. Larvae with two resistance alleles in any combination were resistant, whereas those with one or none were susceptible to Cry1Ac. Together with previous evidence, the results reported here identify the cadherin gene as a leading target for DNA-based screening of resistance to Bt crops in lepidopteran pests.
Because of their low toxicity to vertebrates and most other nontarget organisms, insecticidal crystal proteins from Bacillus thuringiensis (Bt) are environmentally friendly alternatives to conventional insecticides (1). Bt toxins kill insects by binding to specific target sites and disrupting midgut membranes (1). Transgenic crops producing Bt toxins that kill lepidopteran larvae are grown on millions of hectares, but evolution of resistance by pests could cut short their success (2–5). So far, no field outbreaks of resistance have occurred in response to Bt crops (5, 6). However, field populations of the diamondback moth (Plutella xylostella) have evolved resistance to Bt sprays and laboratory strains of many pests have been selected for resistance to Bt toxins (2, 3).
Concerns about resistance to Bt crops led the U.S. Environmental Protection Agency to mandate the “refuge strategy” for delaying pest resistance (7). Thus, farmers growing cotton that produces Bt toxin Cry1Ac must plant refuges of non-Bt cotton to enable survival of susceptible pests. The refuge strategy is expected to be most effective when resistance to Bt cotton is inherited as a recessive trait. Ideally, rare homozygous resistant pests (with two resistance alleles) emerging from Bt cotton mate with relatively abundant homozygous susceptible pests (with no resistance alleles) from refuges. If resistance is recessive, Bt cotton kills heterozygous progeny (with only one resistance allele) produced by such matings. Models predict that under these conditions, refuges can delay resistance substantially (4, 8).
Tests of the refuge strategy have been problematic because of the difficulty of monitoring resistance in the field, especially when resistance is recessive and rare. Bioassays have been used to estimate resistance allele frequencies, but their failure to distinguish between heterozygotes and susceptible homozygotes usually necessitates huge sample sizes or multigenerational experiments to detect rare resistant homozygotes (9–11). DNA-based detection of resistance alleles in heterozygotes could increase efficiency >1,000-fold compared with conventional bioassays (11), yet such screening requires knowledge of the molecular genetic basis of resistance to Bt toxins in field populations.
The most common type of lepidopteran resistance to Bt toxins (called “mode 1”) entails a high level of resistance to at least one Cry1A toxin, recessive inheritance, reduced binding of at least one Cry1A toxin, and little or no cross-resistance to Cry1C (12). Mode 1 resistance occurs in some strains of diamondback moth, Indianmeal moth (Plodia interpunctella), tobacco budworm (Heliothis virescens), and pink bollworm (Pectinophora gossypiella) (refs. 12 and 13, and J. González-Cabrera, B. Escriche, B.E.T., and J. Ferré unpublished data). The simplest explanation for mode 1 resistance is that modifications of target sites reduce or eliminate binding of Cry1A toxins in homozygous resistant individuals, but have little effect on susceptibility of heterozygous individuals to Cry1A toxins. Such modifications would also have little effect on susceptibility of resistant homozygotes to toxins such as Cry1C that attack target sites independent from Cry1A receptors (2, 3). The two major candidates for targets of Cry1A toxins are aminopeptidases and cadherins, both of which bind Cry1A toxins in Lepidoptera (14–18).
Genetic mapping experiments with the laboratory-selected YHD2 strain of H. virescens, a major cotton pest, showed tight linkage between resistance to Cry1Ac and a cadherin-encoding gene (called BtR-4 or HevCaLP), but not to genes encoding aminopeptidases (19). Insertion of a retrotransposon disrupts BtR-4 in the YHD2 strain, which has >10,000-fold resistance to Cry1Ac (19). Complementation tests using crosses between field-collected males and YHD2 females suggest that BtR-4 resistance alleles occur in field populations (9, 19), but the resistance allele in the YHD2 strain has not yet been detected in the field or associated with survival on Bt cotton.
If mutations in cadherin genes are the primary basis of mode 1 resistance in the field, focusing on these genes could accelerate progress in resistance monitoring and management. Here we tested the hypothesis that mode 1 resistance to Bt toxin Cry1Ac is linked to the cadherin gene in pink bollworm, a worldwide cotton pest. We report that field-derived strains of pink bollworm harbored three cadherin alleles associated with resistance to Cry1Ac and survival on Bt cotton.
Materials and Methods
Insect Strains.
We used six strains of pink bollworm: a susceptible strain (APHIS-S), four laboratory-selected Bt-resistant strains (AZP-R, SAF97-R, MOV97-R10, and TX01-R), and an unselected heterogeneous strain (TX01). Bioassays indicated that major alleles for Cry1Ac resistance were rare or absent in APHIS-S, which had been reared in the laboratory for >20 years without exposure to insecticides (20). AZP-R was started by pooling survivors of exposure to various concentrations of Cry1Ac in diet from 10 strains derived in 1997 from Arizona cotton fields (20, 21). AZP-R was further selected by using 10 or 100 μg of Cry1Ac per ml diet, leading to 3,100-fold resistance to Cry1Ac (13). Two of the strains contributing individuals to AZP-R were SAF97 and MOV97, which were started in 1997 from individuals collected in Safford and Mohave Valley, respectively (20). The TX01 strain was derived from 24 individuals collected from Tornillo, Texas, in 2001. SAF97, MOV97, and TX01 were reared without exposure to Cry1Ac. These three strains had a high proportion of susceptible individuals, but also had some resistant individuals (ref. 20 and results reported here). A subset of each of these three heterogeneous strains was selected with 10 μg Cry1Ac per ml diet to produce the resistant strains SAF97-R, MOV97-R10 (22, 23), and TX01-R, respectively. All strains were reared in the laboratory on wheat germ diet (21).
RNA and Reverse Transcription–PCR of the Pink Bollworm Cadherin Gene.
PCR with partially degenerate primers based on H. virescens BtR-4 (19) amplified a product from APHIS-S genomic DNA containing two exons and an intron in the predicted positions. The exon sequence was identical to region 4,933–4,986 of the pink bollworm cadherin cDNA sequence (BT-R2) from an unnamed susceptible strain (18). Based on the observed identity, we used the BT-R2 sequence to design specific primers for isolating the coding sequence of the cadherin gene from our susceptible and resistant strains of pink bollworm. Specific primers divided the gene into three overlapping fragments: −106 (in the 5′ untranslated region) to 1441, 1381–3645, and 3221 to the poly(A) tail (see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org).
Total RNA was extracted with TRIzol reagent (GIBCO/BRL) according to the manufacturer's instructions and reverse transcribed with SuperScript II RNase H− reverse transcriptase (Invitrogen). The cDNA fragments served as templates for subsequent PCR amplification with denaturation at 94°C for 3 min, followed by 35 cycles at 94°C for 1 min, at 55°C for 1 min, and at 72°C for 2 min. PCR products of the expected sizes were excised and purified by using the QIAquick gel extraction kit (Qiagen, Valencia, CA) and cloned by using the pGEM-T easy vector system (Promega). At least three clones for each fragment were fully sequenced in both strands. DNA sequence files were visualized by using chromas version 1.45 (Technelysium Pty, Helensvale, Australia) and analyzed by using dnaman (Lynnon BioSoft, Montreal) software.
DNA Purification.
Individual larvae or pupae were put on parafilm stretched over a hard surface. After grinding in 50 μl of cold lysis buffer (5 mM Tris-HCl, pH 8.0, containing 0.5 mM EDTA, 0.5% Nonidet P-40, and 1 mg/ml proteinase K), the extracts were incubated at 65°C for 30 min, and at 95°C for 10 min before PCR amplification (as described above).
Diet Bioassays.
We used two types of diet bioassays: survival and growth bioassays. In both types, neonates were tested individually in the dark at 29°C (±2°C) and the source of Cry1Ac was MVPII (Dow Agrosciences), which is identical to holotype Cry1Ac in the active region of the toxin (23). In survival bioassays, larvae were fed diet with 10 μg of Cry1Ac per ml of diet for 21 days (20). Adjusted survival was calculated as survival of larvae on treated diet divided by survival of larvae on untreated diet × 100%. In growth bioassays, larvae were weighed after eating diet with 1 μg of Cry1Ac per ml for 11 days (13). The high concentration and long duration of the survival bioassay diagnose a high level of resistance (13, 20, 24). In the growth bioassay, resistant larvae grow faster than others, but the lower concentration and shorter duration enable recovery of most susceptible as well as resistant individuals, thus providing larger sample sizes and more powerful tests for analyzing inheritance (13).
For linkage analysis (see below), at the end of both bioassays, survivors were weighed and reared to pupation on untreated diet. Survivors (backcross progeny), their parents (F1 and AZP-R), and grandparents (APHIS-S and AZP-R) were frozen individually in 95% ethanol at −70°C for DNA testing.
Linkage Analysis.
To generate informative families of pink bollworm for biphasic linkage analysis (25), we conducted single-pair crosses (13). We crossed the susceptible APHIS-S strain with the resistant AZP-R strain to produce F1 progeny and separately backcrossed male and female F1 progeny to AZP-R (Fig. 1). Resistance of AZP-R to Cry1Ac is functionally recessive and controlled primarily by a major locus (13). Previous analysis of 696 larvae from 12 backcross families tested with the growth bioassay revealed a bimodal weight distribution consistent with the expected 1:1 ratio of resistant (rr) to susceptible (rs) larvae (13).
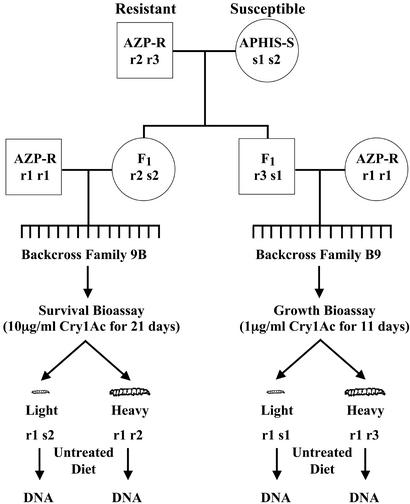
Figure 1.
Linkage analysis. DNA from two backcross families (9B and B9) was analyzed to test for linkage between the cadherin gene and resistance to Cry1Ac. A resistant male from AZP-R was crossed with a susceptible female from APHIS-S to create F1 family B. A female from F1 family B was backcrossed to a male from AZP-R family 9 to create backcross family 9B. One of her brothers was backcrossed to a female from AZP-R family 9 to create backcross family B9. After exposure to Cry1Ac in bioassays, survivors were weighed, reared to pupation on untreated diet, and frozen for DNA analysis. DNA screening of 62 backcross progeny (22 from family 9B and 40 from family B9) showed that all resistant (heavy) individuals were rr, whereas all susceptible (light) individuals were rs.
We screened the DNA of parents of the 12 backcross families to find families in which markers at the cadherin locus would enable a test for linkage. The linkage analysis reported here focuses on two such backcross families (9B and B9, Fig. 1). An AZP-R male and an APHIS-S female were crossed to produce F1 family B. From F1 family B, a female was backcrossed to an AZP-R male from family 9 to create family 9B. One of her brothers was backcrossed to an AZP-R female from family 9 to create family B9. For backcross family 9B, we screened DNA of survivors from the survival bioassay. Because crossing over occurs only in male Lepidoptera and the father of family 9B was resistant (rr), examining this family tested the hypothesis that the cadherin locus and genetic control of resistance to Cry1Ac are on the same chromosome. Backcross family B9, in which the father was an F1 (rs), was tested with the growth bioassay to determine how tightly resistance is linked to the cadherin locus. The larger sample size afforded by the growth bioassay was essential for detecting potential crossing over between the cadherin gene and resistance loci in the F1 father.
Larvae weighing >10 mg in the survival bioassay or >20 mg in the growth bioassay were designated as heavy (putative rr), the others were designated as light (putative rs). To determine whether resistance was genetically linked with the cadherin locus, we tested the association between phenotype (heavy vs. light) and cadherin genotype.
Greenhouse Bioassays.
We used greenhouse bioassays (20, 23, 26, 27) to assess the association between cadherin genotype and survival on cotton plants. Paper towels bearing eggs laid by AZP-R females in the laboratory were divided into pieces (1 cm2), each with 40 eggs. Each piece was randomly assigned to one of three treatments: control, Bt cotton, or non-Bt cotton. To estimate cadherin genotype frequencies in AZP-R before the greenhouse bioassays, control eggs were allowed to hatch and neonates were frozen for DNA analysis. To infest plants, we put the remaining eggs under the bracts of selected bolls. On May 14, 2002, we infested 100 bolls on 20 Bt cotton plants (Deltapine 33B) and 50 bolls on 10 non-Bt cotton plants (Deltapine 5415). Infested bolls were enclosed in screened cages to capture survivors that emerged from bolls. After 3 weeks, cages were checked every 2 days for individuals that emerged from bolls, which were allowed to pupate and were frozen for DNA analysis. After 7 weeks, bolls were checked for larvae and pupae. Individuals that reached the fourth instar by 7 weeks were scored as survivors. Survival percentage was estimated as the number of survivors divided by the number of entrance holes × 100%. For individuals tested on plants, DNA was analyzed only from those that reached the pupal stage.
Results
Cadherin Alleles from Susceptible and Resistant Pink Bollworm.
Cadherin cDNA isolated from the susceptible APHIS-S strain had 5,208 bp encoding a predicted protein of 1,735 aa (Fig. 2), which we call BtR (for Bt resistance, GenBank accession no. AY198374). Similar to other lepidopteran cadherins (17–19, 28), the proposed structure of BtR includes a putative membrane signal sequence, 11 extracellular cadherin repeats, a membrane-proximal extracellular domain, a transmembrane domain, and a cytoplasmic domain (Fig. 2). Amino acid identity of BtR to other lepidopteran cadherins is 60% for Bombyx mori (BtR175, GenBank accession no. BAA77212), 58% for Manduca sexta (BT-R1, GenBank accession no. AF319973), and 55% for H. virescens (HevCaLP, GenBank accession no. AF367362).
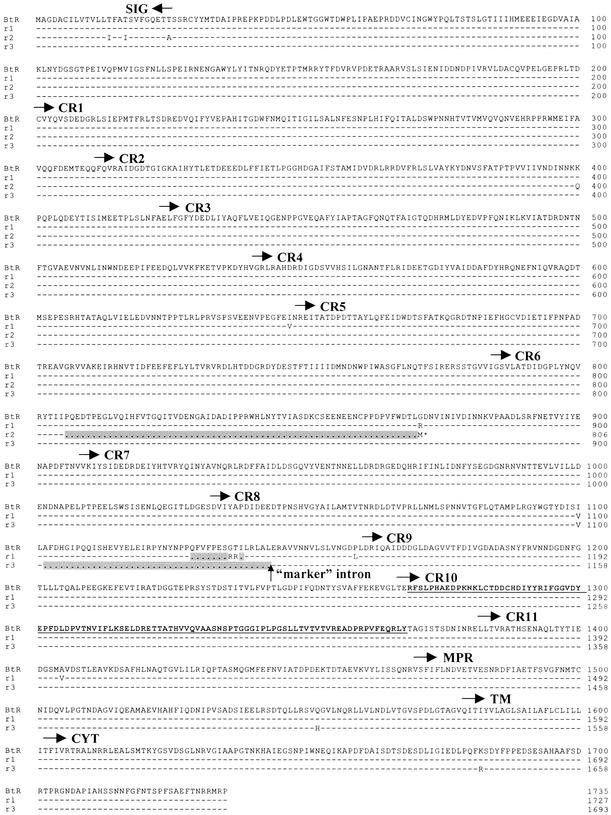
Figure 2.
Amino acid sequence of BtR (from the susceptible APHIS-S strain) and resistance alleles r1, r2, and r3 deduced from cloning and sequencing cDNA. Identical residues are designated by dashes. Deleted amino acids are indicated by dots and gray background. An asterisk shows the premature stop codon in r2. Protein sequence analysis was done by using the Institut Suisse de Recherches Experimentales sur le Cancer (Lausanne, Switzerland) ProfileScan server (http://hits.isb-sib.ch/cgi-bin/PFSCAN) and the Simple Modular Architecture Research Tool (http://smart.ox.ac.uk). Horizontal arrows specify start sites of putative domains (33). SIG, signal peptide; CR, cadherin repeat; MPR, membrane-proximal region; TM, transmembrane domain; CYT, cytoplasmic domain. The vertical arrow indicates the position of the “marker” intron. Bold, underlined amino acids show the putative binding region (18).
Compared with BT-R2, the cadherin previously reported for susceptible pink bollworm (18), the cDNA sequence of BtR from APHIS-S is 99% identical from nucleotides 1 to 5031, with no deletions or insertions, 31 nucleotide substitutions, and 12 amino acid substitutions. However, from nucleotide 5032 to the end of the coding sequence, amino acid identity is only 8%. In this region, amino acid identity with cadherins from M. sexta, B. mori, and H. virescens is 32–43% for BtR, but only 4–7% for BT-R2, which might reflect a sequencing error in BT-R2.
We identified three alleles (r1, r2, and r3) of the BtR gene in the resistant AZP-R strain, each with a unique major deletion (Fig. 2). The r1 allele lacks 24 bp (nucleotides 3386–3407 and 3413–3414) resulting in two amino acid substitutions (G1136R and T1137R) and omission of eight amino acids (1129–1135 and 1138). The r2 allele has a 202-bp deletion (2415–2616) creating a frame shift that introduces a premature stop codon after amino acid 805. The r3 allele has a 126-bp deletion (3302–3427) that eliminates 42 amino acids (1103–1144). No other insertions, deletions, or premature stop codons occurred in these three r alleles. Moreover, we did not find any amino acid substitution that occurred in all three r alleles.
All individuals tested from AZP-R (n > 150) had two copies of r alleles (r1r1, r1r2, r1r3, r2r2, r2r3, or r3r3). Two other resistant Arizona strains tested had high frequencies of r alleles, with r1 and r2 in SAF97-R and r1 and r3 in MOV97-R10. In contrast, none of the three r alleles occurred in 50 individuals from APHIS-S.
Linkage Analysis.
We tested for genetic linkage between BtR and resistance to Cry1Ac by using backcross (F1 × AZP-R) families (13). The intron adjacent to the r1 deletion (between nucleotides encoding amino acids Leu-1143 and Glu-1144) served as a genetic marker. In the first 540 bp of this “marker” intron, each r allele in AZP-R has a unique pattern of 24 polymorphic sites (mr1, mr2, and mr3; Table 1), which facilitated efficient genotyping by direct sequencing of PCR products.
Table 1.
Polymorphic nucleotides in the “marker” intron used to identify cadherin alleles
| Marker | 31 | 92 | 143 | 183 | 196 | 200 | 221–224 | 284 | 380 | 381 | 383 | 408 | 419 | 429 | 434 | 440 | 447 | 461–463 | 488 | 513 | 520 | 530 | 538 | 540 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mr1 | A | A | C | G | T | A | … | G | C | A | T | C | T | A | T | C | A | … | G | G | C | C | T | G |
| mr2 | C | G | C | G | G | A | … | G | A | G | T | C | C | C | C | T | T | AAC | A | A | T | T | G | C |
| mr3 | C | G | A | A | G | G | … | A | C | G | G | T | C | C | C | C | T | AAC | A | A | C | C | T | G |
| ms1 | C | G | C | A | G | G | TAAG | G | C | G | T | C | C | C | C | C | T | AAC | A | A | C | C | T | G |
| ms2 | A | A | C | G | T | A | … | G | C | A | T | C | T | A | T | C | A | … | G | G | C | C | T | G |
This intron occurs between nucleotides encoding amino acids 1143 and 1144 (Fig. 2). The first 540 bp of the intron were amplified. PCR products were recovered from agarose gels using the QIAquick gel extraction kit (Qiagen) and sequenced. Diagnostic nucleotides are shown in boldface. Dots indicate deletions.
Screening DNA from the parents of 12 backcross families identified two informative families (9B and B9) in which various r and s alleles segregated (Fig. 1). The AZP-R grandfather was r2r3 and the APHIS-S grandmother had two alleles, designated s1 and s2, that differ from each other and from r2 and r3 in marker intron sequence (ms1 and ms2, Table 1). Alleles r1 and s2, which have the same marker intron sequence (Table 1), were distinguished by the 24-bp deletion in the coding region that occurred in r1 but not in s2. For family 9B, the AZP-R father was r1r1 and the F1 mother was r2s2. For family B9, the AZP-R mother was r1r1 and the F1 father was r3s1. As expected, the weight distribution of larvae fed diet with Cry1Ac was bimodal for both of these backcross families (Fig. 3).
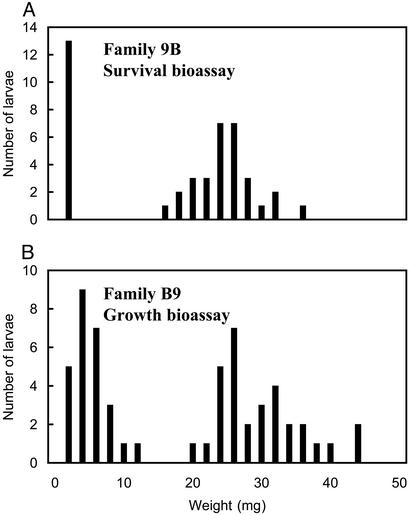
Figure 3.
Weight distribution of backcross larvae tested in bioassays. (A) Backcross family 9B was tested with the survival bioassay (10 μg of Cry1Ac per ml of diet for 21 days). Of 58 larvae tested, 30 were heavy (>10 mg), 13 were light, and 15 died. (B) Family B9 was tested with the growth bioassay (1 μg of Cry1Ac per ml of diet for 11 days). Of 60 larvae tested, 30 were heavy (>20 mg), 27 were light, and 3 died.
To test for linkage, we determined the BtR genotype for 11 heavy (putative rr) and 11 light (putative rs) individuals from family 9B as well as 20 heavy and 20 light individuals from family B9. In family 9B, all 11 heavy progeny were r1r2 whereas all 11 light progeny were r1s2 (χ2 = 22, df = 1, P < 0.0001). Analogously, in family B9, all heavy progeny were r1r3 and all light progeny were r1s1 (χ2 = 40, df = 1, P < 0.0001). Because crossing over occurs only in males in Lepidoptera, the results from family 9B (in which the father was r1r1) indicate linkage between resistance and the chromosome carrying BtR. The results from family B9 (in which the father was r3s1) demonstrate tight linkage (no observed recombinants) between resistance and BtR.
Association Between Cadherin Alleles and Resistance in a Heterogeneous Strain from Texas.
We used survival and growth bioassays and a selection experiment to determine whether r1, r2, or r3 were associated with Cry1Ac resistance in a heterogeneous strain (TX01) derived in 2001 from a cotton field in Texas. This heterogeneous strain had been reared in the laboratory for nine generations without exposure to Cry1Ac.
In survival bioassays (21 days on diet with 10 μg Cry1Ac per ml diet), adjusted survival of TX01 was 14% (n = 650). We determined the BtR genotype for nine survivors from this diagnostic test. Five were r3r3 and four were r1r3. In addition, we tested neonates with growth bioassays (11 days on diet with 1 μg Cry1Ac per ml), weighed them, and determined the BtR genotype of six heavy larvae (11–50 mg, mean = 29.5 mg) and 20 light larvae (<6 mg, mean = 1.1 mg). Of the six heavy larvae, four were r1r3 and two were r3r3. Of the 20 light larvae, 12 had one r allele (4 had r1, 8 had r3) and 8 had none.
We selected the TX-01 strain for resistance by feeding 540 neonates of the F10 generation on diet with 10 μg Cry1Ac per ml for 21 days. Survivors of this single generation of selection were pooled and allowed to mate to begin the TX01-R strain. All 20 larvae tested from the first generation progeny of these survivors were rr: nine were r1r1, ten were r1r3, and one was r3r3. Consistent with recessive inheritance of resistance to Cry1Ac in pink bollworm (13, 20, 26, 24), these results show that in TX01 and TX01-R, larvae with two copies of r alleles were resistant, whereas those with one r allele or none were susceptible.
Greenhouse Bioassays.
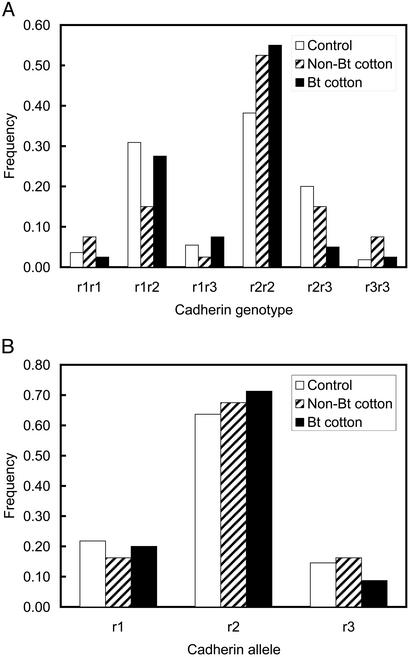
In greenhouse bioassays with the AZP-R strain, all six resistant genotypes (r1r1, r1r2, r1r3 r2r2, r2r3, and r3r3) were represented among survivors of Bt cotton (Fig. 4). The frequency of the three r alleles and the six rr genotypes did not vary significantly among a control group of neonates, survivors on Bt cotton, and survivors on non-Bt cotton (Fig. 4). Thus, we did not detect variation among alleles or among rr genotypes in survival on Bt cotton or on non-Bt cotton. Further, on either Bt cotton or non-Bt cotton, pupal weight and time to pupation did not vary significantly among the six rr genotypes (one-way ANOVA, df = 5, 34, P > 0.27 in each case). These results show that some individuals with any of the six rr genotypes can survive on Bt cotton and on non-Bt cotton, yet they do not exclude minor or moderate differences in performance among genotypes.
Figure 4.
Greenhouse bioassays with the resistant AZP-R strain of pink bollworm. (A) Cadherin genotype frequencies of larvae (number of individuals = 55 for control, 40 for survivors on non-Bt cotton, and 40 for survivors on Bt cotton). Control genotype frequencies did not deviate significantly from Hardy–Weinberg equilibrium (χ2 = 0.58, df = 5, P = 0.99). (B) Cadherin allele frequencies of larvae (number of alleles = 110 for control, 80 for survivors on non-Bt cotton, and 80 for survivors on Bt cotton).
Survival was 4.7% (92 of 1,941) on Bt cotton and 10.9% (108 of 907) on non-Bt cotton, which yields an adjusted survival on Bt cotton of 43.3% (4.7 divided by 10.9), similar to previous results (refs. 20, 26, and 27, but also see ref. 23).
Discussion
Several lines of evidence support the hypothesis that the r1, r2, and r3 alleles of the pink bollworm cadherin gene BtR confer recessively inherited resistance to Bt toxin Cry1Ac. First, screening of four strains from Arizona showed that these alleles occurred at high frequency in three resistant strains but not in a susceptible strain. Of >150 individuals tested from the AZP-R strain, which has 3,100-fold resistance to Cry1Ac, all were rr (r1r1, r1r2, r1r3, r2r2, r2r3, or r3r3). Second, in tests of 62 backcross progeny from a genetic linkage analysis with AZP-R and the susceptible APHIS-S strain, all resistant larvae were rr, whereas all susceptible larvae were rs. Thus, the BtR locus is tightly linked with Cry1Ac resistance in the AZP-R strain. Third, of 35 larvae tested from the heterogeneous TX01 strain recently derived from the field in Texas, all 15 resistant larvae were rr, whereas all 20 susceptible larvae had either one r allele or none. Fourth, all 20 larvae tested from the resistant TX01-R strain, derived by a single selection of the TX01 strain, were rr.
Although the results described above demonstrate a strong association between resistance and the three r alleles, functional studies of the proteins encoded by the r alleles are needed to definitively determine their effect on susceptibility to Cry1Ac. Some additional evidence available now suggests, but does not prove, a causal connection. Each of the r alleles has a deletion in a gene encoding cadherin protein, which binds Cry1Ac in pink bollworm (18). All three deletions are upstream of the DNA encoding the putative toxin-binding region of cadherin (17, 18). The stop codon introduced by the 202-bp deletion in r2 is expected to block production of the binding region. The predicted amino acid deletions associated with r1 (8 aa) and r3 (42 aa) are ≈100 aa upstream of the putative binding region (Fig. 2).
We hypothesize that the deletions in r1, r2, and r3 interfere with toxicity of Cry1Ac and thereby confer resistance in homozygous resistant individuals lacking the susceptible form of cadherin protein. This parallels the mechanism proposed for the YHD2 strain of H. virescens, in which disruption of a related cadherin gene is tightly linked with resistance to Cry1Ac (19). In H. virescens and pink bollworm, reduced binding of one or more Cry1A toxins to brush border membrane vesicles is associated with resistance (refs. 29 and 30, and J. González-Cabrera, B. Escriche, B.E.T., and J. Ferré, unpublished data).
The three pink bollworm resistance alleles identified here each harbor a unique deletion, whereas the resistance allele in the YHD2 strain of H. virescens has an insertion (19). Thus, at least four different mutations of the cadherin gene that prevent production of a full-length protein are associated with Cry1Ac resistance. This variation among resistance alleles differs from some well documented cases of insecticide resistance. For example, replacement of a single amino acid (alanine-302) encoded by the Rdl gene is associated with cyclodiene resistance in a wide range of insects (31), and a single overtranscribed resistance allele of the DDT-R gene occurs globally in Drosophila melanogaster (32). Because a limited number of Bt-resistant strains of pink bollworm and H. virescens have been tested so far, we suspect that more extensive screening will uncover additional cadherin resistance alleles.
The four known cadherin resistance alleles are recessive, showing that production of some full-length protein in heterozygotes is sufficient for cadherin's role in the mode of action of Cry1Ac. Although many cadherins mediate cell–cell adhesion in vertebrates (33), their normal function in Lepidoptera is not known. Whereas mutational disruption of cadherins in humans is associated with cancer (33), fitness costs associated with Bt resistance in pink bollworm suggest that its cadherin resistance mutations reduce overwintering survival and survival on non-Bt cotton (22, 34).
Resistance to Cry1Ac in both the pink bollworm and the YHD2 strain of H. virescens fits the mode 1 profile and is linked to the cadherin locus. We hypothesize that cadherin mutations are also associated with mode 1 resistance in other lepidopteran pests, such as diamondback moth and Indianmeal moth. Conversely, other types of resistance (3, 12) are likely to involve other loci.
Although results reported here for pink bollworm and previously for H. virescens provide strong evidence that cadherin mutations are associated with Cry1Ac resistance, a crucial practical issue is whether such mutations enable survival on Bt cotton plants. For pink bollworm, putative ss and rs individuals had essentially no survival on Bt cotton plants in previous tests (20, 26, 27). In contrast, results reported here show that rr larvae from the AZP-R strain had 43.3% survival on Bt cotton relative to non-Bt cotton. All AZP-R individuals tested had two r alleles, and we did not detect variation in survival on Bt cotton among the six rr genotypes. Therefore, we cannot exclude the possibility that other genes, environmental factors, or both contributed to variation in survival of AZP-R on Bt cotton. More work is needed to elucidate these additional factors and their potential interactions with cadherin genotype. Nonetheless, our results show that in pink bollworm, cadherin alleles are associated with survival on Bt cotton as well as resistance to Cry1Ac in diet. Thus, the cadherin locus is a prime target for developing DNA-based screening of resistance to Bt crops in field populations of lepidopteran pests.
Supplementary Material
Acknowledgments
We thank S. Borquist, S. Czech, and the staffs of the Extension Arthropod Resistance Management Laboratory and the Arizona Cotton Research and Protection Council for technical assistance, and J. Ferré, B. Oppert, and Y. Park for helpful comments on the manuscript. This work was supported by Vaadia–Binational Agricultural Research and Development Fund Postdoctoral Fellowship FI-300-2000, the University of Arizona, Cotton Inc., the Arizona Cotton Research and Protection Council, Monsanto, the Cotton Foundation, U.S. Department of Agriculture–National Research Initiative Grants 99-35302-8300 and 2001-35302-09976, and U.S. Department of Agriculture Biotechnology Risk Assessment Grant 2001-33120-11213.
Abbreviation
- Bt
Bacillus thuringiensis
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY198374).
References
- 1.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Microbiol Mol Biol Rev. 1998;62:755–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabashnik B E. Annu Rev Entomol. 1994;39:47–79. [Google Scholar]
- 3.Ferré J, Van Rie J. Annu Rev Entomol. 2002;47:501–533. doi: 10.1146/annurev.ento.47.091201.145234. [DOI] [PubMed] [Google Scholar]
- 4.Gould F. Annu Rev Entomol. 1998;43:701–726. doi: 10.1146/annurev.ento.43.1.701. [DOI] [PubMed] [Google Scholar]
- 5.Shelton A M, Zhao J-Z, Roush R T. Annu Rev Entomol. 2002;47:845–881. doi: 10.1146/annurev.ento.47.091201.145309. [DOI] [PubMed] [Google Scholar]
- 6.Carrière Y, Ellers-Kirk C, Sisterson M, Antilla L, Whitlow M, Dennehy T J, Tabashnik B E. Proc Natl Acad Sci USA. 2003;100:1519–1523. doi: 10.1073/pnas.0436708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Environmental Protection Agency. Bt Plant-Pesticides Biopesticides Registration Action Document–Insect Resistance Management. Washington, DC: Environmental Protection Agency; 2000. www.epa.gov/oscpmont/sap/2000/october/brad4_irm.pdf , www.epa.gov/oscpmont/sap/2000/october/brad4_irm.pdf. . [Google Scholar]
- 8.Carrière Y, Tabashnik B E. Proc R Soc London B. 2001;268:1475–1480. doi: 10.1098/rspb.2001.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gould F, Anderson A, Jones A, Sumerford D, Heckel D G, Lopez J, Micinski S, Leonard R, Laster M. Proc Natl Acad Sci USA. 1997;94:3519–3523. doi: 10.1073/pnas.94.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andow D A, Alstad D N, Pang Y-H, Bolin P C, Hutchison W D. J Econ Entomol. 1998;91:579–584. [Google Scholar]
- 11.Tabashnik B E. Proc Natl Acad Sci USA. 1997;94:3488–3490. doi: 10.1073/pnas.94.8.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabashnik B E, Liu Y-B, Malvar T, Heckel D G, Masson L, Ferré J. Philos Trans R Soc London B. 1998;353:1751–1756. [Google Scholar]
- 13.Tabashnik B E, Liu Y-B, Dennehy T J, Sims M A, Sisterson M S, Biggs R W, Carrière Y. J Econ Entomol. 2002;95:1018–1026. doi: 10.1603/0022-0493-95.5.1018. [DOI] [PubMed] [Google Scholar]
- 14.Knight P J K, Crickmore N, Ellar D. Mol Microbiol. 1994;11:429–436. doi: 10.1111/j.1365-2958.1994.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangadala S, Walters F S, English L H, Adang M. J Biol Chem. 1994;269:10099–10092. [PubMed] [Google Scholar]
- 16.Vadlamudi R K, Weber E, Ji T H, Bulla L A., Jr J Biol Chem. 1995;270:5490–5494. doi: 10.1074/jbc.270.10.5490. [DOI] [PubMed] [Google Scholar]
- 17.Nagamatsu Y, Koike T, Sasaki K, Yoshimoto A, Furukawa Y. FEBS Lett. 1999;460:385–390. doi: 10.1016/s0014-5793(99)01327-7. [DOI] [PubMed] [Google Scholar]
- 18.Bulla L A, Jr, Candas M. Pectinophora gossypiella (Pink Bollworm) Bacillus thuringiensis Toxin Receptor BT-R2. Geneva: World Intellectual Property Organization; 2001. , Publication no. WO 01/34807 A2. [Google Scholar]
- 19.Gahan L J, Gould F, Heckel D G. Science. 2001;293:857–860. doi: 10.1126/science.1060949. [DOI] [PubMed] [Google Scholar]
- 20.Tabashnik B E, Patin A L, Dennehy T J, Liu Y-B, Carrière Y, Sims M A, Antilla L. Proc Natl Acad Sci USA. 2000;97:12980–12984. doi: 10.1073/pnas.97.24.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patin A L, Dennehy T J, Sims M A, Tabashnik B E, Liu Y B, Gouge D, Henneberry T J, Staten R. Proc. 1999 Beltwide Cotton Conf. Vol. 2. 1999. pp. 991–996. [Google Scholar]
- 22.Carrière Y, Ellers-Kirk C, Patin A L, Sims M A, Meyer S, Liu Y-B, Dennehy T J, Tabashnik B E. J Econ Entomol. 2001;94:935–941. doi: 10.1603/0022-0493-94.4.935. [DOI] [PubMed] [Google Scholar]
- 23.Tabashnik B E, Dennehy T J, Sims M A, Larkin K, Head G P, Moar W J, Carrière Y. Appl Environ Microbiol. 2002;68:3790–3794. doi: 10.1128/AEM.68.8.3790-3794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y-B, Tabashnik B E, Meyer S, Carrière Y, Bartlett A C. J Econ Entomol. 2001;94:248–252. doi: 10.1603/0022-0493-94.1.248. [DOI] [PubMed] [Google Scholar]
- 25.Heckel D G, Gahan L J, Liu Y-B, Tabashnik B E. Proc Natl Acad Sci USA. 1999;96:8373–8377. doi: 10.1073/pnas.96.15.8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y B, Tabashnik B E, Dennehy T J, Patin A L, Bartlett A C. Nature. 1999;400:519. doi: 10.1038/22919. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y-B, Tabashnik B E, Dennehy T J, Patin A L, Sims M A, Meyer S, Carrière Y. J Econ Entomol. 2001;94:1237–1242. doi: 10.1603/0022-0493-94.5.1237. [DOI] [PubMed] [Google Scholar]
- 28.Dorsch J A, Candas M, Griko N B, Maaty W S A, Midboe E G, Vadlamudi R K, Bulla L A., Jr Insect Biochem Mol Biol. 2002;32:1025–1036. doi: 10.1016/s0965-1748(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 29.Lee M K, Rajamohan F, Gould F, Dean D H. Appl Environ Microbiol. 1995;63:2218–2213. doi: 10.1128/aem.61.11.3836-3842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurat-Fuentes J L, Gould F, Adang M. Appl Environ Microbiol. 2002;68:5711–5717. doi: 10.1128/AEM.68.11.5711-5717.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ffrench-Constant R H, Anthony N, Aronstein A, Rocheleau T, Stilwell G. Annu Rev Entomol. 2000;48:449–466. doi: 10.1146/annurev.ento.45.1.449. [DOI] [PubMed] [Google Scholar]
- 32.Daborn P J, Yen J L, Bogwitz M R, Le Goff G, Feil E, Jeffers S, Tijet N, Perry T, Heckel D, Batterham P, et al. Science. 2002;297:2253–2256. doi: 10.1126/science.1074170. [DOI] [PubMed] [Google Scholar]
- 33.Nollet F, Kools P, van Roy F. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 34.Carrière Y, Ellers-Kirk C, Liu Y B, Sims M A, Patin A L, Meyer S, Dennehy T J, Tabashnik B E. J Econ Entomol. 2001;94:1571–1576. doi: 10.1603/0022-0493-94.6.1571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.