Abstract
DNA double-strand breaks (DSBs) are generally accepted to be the most biologically significant lesion by which ionizing radiation causes cancer and hereditary disease. However, no information on the induction and processing of DSBs after physiologically relevant radiation doses is available. Many of the methods used to measure DSB repair inadvertently introduce this form of damage as part of the methodology, and hence are limited in their sensitivity. Here we present evidence that foci of γ-H2AX (a phosphorylated histone), detected by immunofluorescence, are quantitatively the same as DSBs and are capable of quantifying the repair of individual DSBs. This finding allows the investigation of DSB repair after radiation doses as low as 1 mGy, an improvement by several orders of magnitude over current methods. Surprisingly, DSBs induced in cultures of nondividing primary human fibroblasts by very low radiation doses (≈1 mGy) remain unrepaired for many days, in strong contrast to efficient DSB repair that is observed at higher doses. However, the level of DSBs in irradiated cultures decreases to that of unirradiated cell cultures if the cells are allowed to proliferate after irradiation, and we present evidence that this effect may be caused by an elimination of the cells carrying unrepaired DSBs. The results presented are in contrast to current models of risk assessment that assume that cellular responses are equally efficient at low and high doses, and provide the opportunity to employ γ-H2AX foci formation as a direct biomarker for human exposure to low quantities of ionizing radiation.
Exposure to ionizing radiation (IR) induces leukemia and other cancers, and damage to DNA in the nucleus of a single cell likely represents an initiating event for carcinogenesis. Estimates of cancer risk from exposure to IR are based on epidemiological studies of exposed human populations, mainly the atomic bomb survivors of Hiroshima and Nagasaki. This approach has provided relatively reliable estimates of risk for high dose and high-dose rate exposures, yet it is the effect of low doses and low-dose rates that is of major importance for the general population. Risk estimates for low doses and an additional factor of 2–10 for low-dose rates are based on extrapolations from existing high-dose data. This model assumes that cellular responses, including DNA repair, operate equally efficient at low and high IR doses (1). DNA double-strand breaks (DSBs) are considered to be the most relevant lesion for the deleterious effects of IR (2, 3), and a single radiation track can produce this kind of damage. All of the experimental data for IR-induced DSBs and their repair, however, have been obtained at high doses of low linear-energy-transfer radiation, where a single cell is traversed by many radiation particles. No information is available for the situation most relevant for public health, where a single electron track impacts on a cell (1).
One of the earliest steps in the cellular response to DSBs is the phosphorylation of serine 139 of H2AX, a subclass of eukaryotic histone proteins that are part of the nucleoprotein structure called chromatin (4). Using a fluorescent antibody specific for the phosphorylated form of H2AX (γ-H2AX), discrete nuclear foci can be visualized at sites of DSBs, either induced by exogenous agents such as IR (5, 6) or generated endogenously during programmed DNA rearrangements (refs. 7–9; see refs. 10 and 11 for review). Initial studies had observed a close correlation between the number of γ-H2AX foci and the number of expected DSBs after irradiation with 0.6 Gy (5). Recently, a direct correlation was observed between the number of foci and the number of DSBs produced by decay of 125I incorporated into cellular DNA (12), suggesting that each focus may represent an individual break and that each DSB may form a focus. The relationship between DSB repair and the disappearance of γ-H2AX foci is less clear. Although the number of foci per cell was shown to decrease with repair time after irradiation with 0.6 Gy (5), this was not the case after irradiation with 12 Gy (13). Additionally, murine cells knocked-out for the gene encoding the DNA-dependent protein kinase catalytic subunit, known to be grossly defective in DSB repair, were analyzed for H2AX phosphorylation by Western blotting, and showed a pattern of γ-H2AX dephosphorylation with repair time similar to repair-proficient cells (6).
In this work, we observed a quantitative similarity between the induction and repair of DSBs determined at higher doses by pulsed-field gel electrophoresis (PFGE) and the formation and disappearance of γ-H2AX foci. This is additional strong evidence that a γ-H2AX focus represents a DSB and shows that γ-H2AX foci formation can be used to measure the repair of individual DSBs in human cells. Thus, in subsequent descriptions, we use foci and DSBs interchangeably. We show that DSBs can be detected after IR doses as low as 1 mGy and provide evidence of a linear relationship between DSB induction and dose between 1 mGy and 100 Gy. We further show that DSBs induced by very low doses remain unrepaired for many days. This finding challenges current models of risk assessment for low IR doses and provides the exciting opportunity to employ γ-H2AX foci formation for monitoring human exposure to low levels of IR.
Materials and Methods
Cell Culture and X-Irradiation.
Primary human fibroblasts from the lung, MRC-5 (wild type; European Collection of Cell Cultures), skin, HSF1, HSF2 (wild type; provided by K. Dittmann, University of Tübingen, Tübingen, Germany), and 180BR (deficient in DNA ligase IV; provided by P. Jeggo, University of Sussex, Falmer, Brighton, U.K.) were grown in MEM supplemented with FCS and antibiotics. All experiments were performed by irradiating nondividing confluent cell cultures with 90 kV x-rays. For doses up to 200 mGy, a filter system composed of a 1-mm copper and a 1-mm aluminum plate was used that results in a dose rate of 6–60 mGy/min (depending on the distance from the source); for the higher doses, only the aluminum plate was used, giving a dose rate of 2 Gy/min. Dose rates were determined with an ionization chamber and by chemical dosimetry. For the colony formation assay, cells were plated in two different dilutions in triplicate. For immunofluorescence, cells were grown and irradiated on coverslips. Control samples were sham-irradiated in all experiments.
PFGE and Immunofluorescence Measurements.
PFGE measurements were performed as described (14–16). Conventional PFGE was applied to obtain the time course for DSB repair after 10 and 80 Gy. Initial numbers of DSBs induced in the dose range between 10 and 80 Gy were obtained with a specialized PFGE assay in which the number of DSBs in specific genomic restriction fragments was evaluated (14, 16) and normalized to the total DNA content of a diploid human G1 cell (6 × 109 base pairs). For immunofluorescence, cells were fixed in 2% paraformaldehyde for 15 min, washed in PBS for 3 × 10 min, permeabilized for 5 min on ice in 0.2% Triton X-100, and blocked in PBS with 1% BSA for 3 × 10 min at room temperature. The coverslips were incubated with anti-γ-H2AX antibody (Trevigen, Gaithersburg, MD) for 1 h, washed in PBS, 1% BSA for 3 × 10 min, and incubated with Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (Molecular Probes) for 1 h at room temperature. Cells were washed in PBS for 4 × 10 min and mounted by using Vectashield mounting medium with 4,6 diamidino-2-phenylindole (Vector Laboratories). Fluorescence images were captured by using a Zeiss Axioskop 2 mot epifluorescent microscope equipped with charge-coupled device camera and isis software (Metasystems, Altlussheim, Germany). Optical sections through the nuclei were captured at 0.2-μm intervals, and the images were obtained by projection of the individual sections. BrdUrd labeling (for 30 min) and detection of BrdUrd-positive cells were performed by using the Cell Proliferation Labeling Reagent and a monoclonal anti-BrdUrd antibody (Amersham Pharmacia Biotech) that was detected by using Alexa Fluor 594-conjugated goat anti-mouse secondary antibody (Molecular Probes). Experiments with secondary antibodies alone were performed to verify the specificity of the signals.
Data Evaluation and Reproducibility.
For quantitative analysis, foci were counted by eye during the microscopic and imaging process by using a ×100 objective. Approximately 1% of the nuclei were substantially larger than normal (possibly indicating the presence of tetraploid or G2-phase cells), and were not considered for evaluation. For each sample, cell counting was performed until at least 40 cells (for high doses) and at least 40 foci (for low doses) were registered. The specific numbers of cells counted per single determination were: control, 0.1, and 0.5 mGy, 600–800 cells; 1.2 mGy, 400–800 cells; 5 mGy, 200–400 cells; 20 mGy, 100–400 cells; 200 mGy, 100–400 cells; and 2 Gy, 40–100 cells. The error bars in Figs. 1E, 3, and 4 represent the SEM from the analysis of these numbers of cells. The experiments shown in Figs. 1 C–E and 3 A and B, were repeated with new cell cultures, and the results from the analysis of a similar number of cells were always within 10% of the mean values presented. The γ-H2AX data in Fig. 2 show the average from two to three independent experiments, and the error bars in Fig. 2 are the SEM from all cells analyzed. The results from PFGE measurements in Figs. 1F and 2 represent the average from two independent experiments. The data points in Figs. 3C and 4 represent single determinations. Cells with apoptotic features (bubble-like appearance of the nucleus) or micronuclei (see Table 1) were not considered for γ-H2AX analysis.
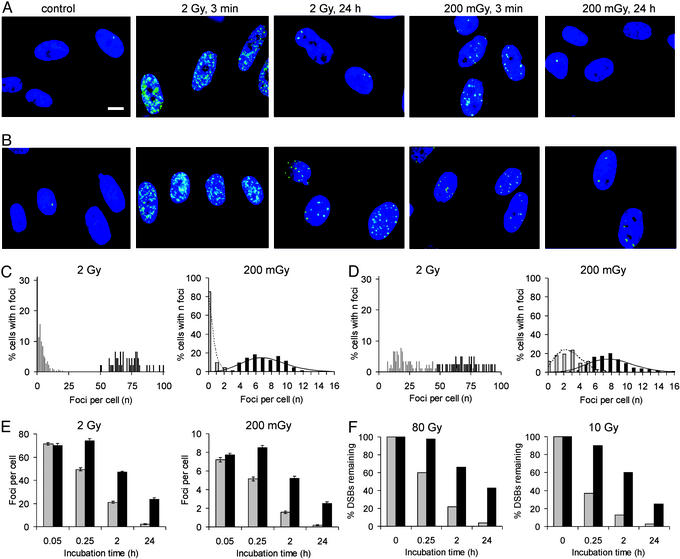
Figure 1.
DSB induction and repair in repair-proficient (MRC-5) and repair-deficient (180BR) primary human fibroblasts. (A) γ-H2AX foci (green) in MRC-5 cells; nuclei were stained with 4,6 diamidino-2-phenylindole (blue); scale bar = 10 μm. (B) γ-H2AX foci in 180BR cells. (C) Distribution of MRC-5 cells with n foci either 3 min (filled columns) or 24 h (shaded columns) after irradiation. (D) Distribution of 180BR cells with n foci either 3 min (filled columns) or 24 h (shaded columns) after irradiation. (E) Mean number of foci per cell for various repair times in irradiated MRC-5 (shaded columns) or 180BR (filled columns) cells. (F) Time course for the repair of DSBs obtained with PFGE measurements in irradiated MRC-5 (shaded columns) or 180BR (filled columns) cells. The dotted and solid lines in C and D represent Poisson distributions with a mean number of foci that was calculated from the experimental distribution of cells with n foci.
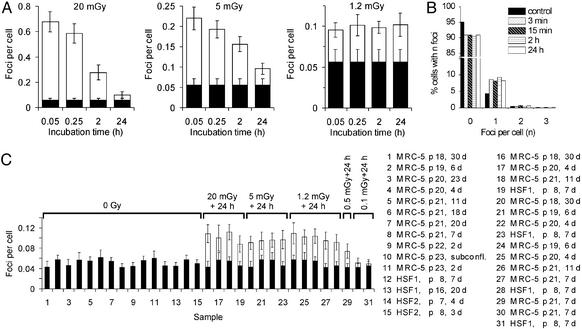
Figure 3.
DSB repair after low doses of IR. (A) Mean number of foci per cell for various repair times in irradiated MRC-5 cells. The IR-induced foci are drawn on top of the background value obtained in a parallel sample (filled columns). (B) Distribution of MRC-5 cells with n foci for an unirradiated sample (filled columns) and for cells irradiated with 1.2 mGy and incubated for various repair times (hatched and open columns). (C) Mean number of foci per cell. The legend indicates the cell line, passage number, and time in confluency before irradiation. The IR-induced foci are drawn on top of the value of the corresponding control sample (filled columns).
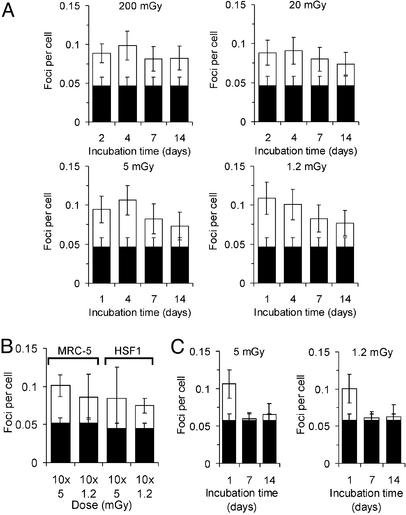
Figure 4.
DSB repair after long incubation times. (A) Mean number of foci per cell for various repair times in irradiated MRC-5 cells. (B) Mean number of foci per cell after repeated daily irradiations of MRC-5 or HSF1 cells. (C) Mean number of foci per cell for various repair times in MRC-5 cells irradiated and allowed to grow. The IR-induced foci are drawn on top of the background values obtained in parallel samples (filled columns).
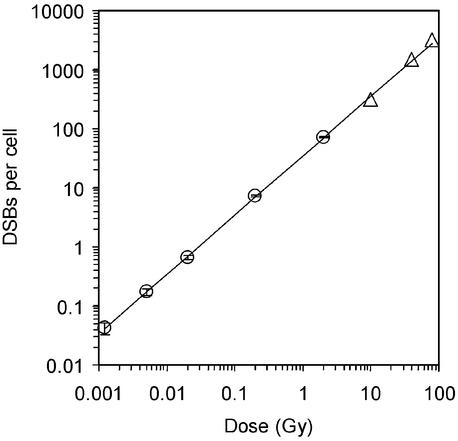
Figure 2.
DSB induction in MRC-5 cells. γ-H2AX foci were counted 3 min after irradiation, and the mean values of foci per cell are shown (circles). Triangles represent DSB induction data obtained from PFGE analysis. The line is a linear fit to the data points with a slope of 35 DSBs per cell per Gy.
Table 1.
Biological effects of very low radiation doses
| Dose, mGy | Cells analyzed | Apoptotic cells | Cells with micronuclei |
|---|---|---|---|
| 0* | 783 | 2 (0.3) | 8 (1.0) |
| 1.2* | 698 | 2 (0.3) | 5 (0.7) |
| 5* | 632 | 2 (0.3) | 7 (1.1) |
| 0† | 622 | 1 (0.2) | 6 (1.0) |
| 1.2† | 676 | 2 (0.3) | 7 (1.0) |
| 5† | 648 | 1 (0.2) | 5 (0.8) |
| 0‡ | 630 | 5 (0.8) | 5 (0.8) |
| 1.2‡ | 641 | 10 (1.6) | 14 (2.2) |
| 5‡ | 646 | 14 (2.2) | 13 (2.0) |
| 0§ | 680 | 4 (0.6) | 11 (1.6) |
| 1.2§ | 619 | 10 (1.6) | 17 (2.8) |
| 5§ | 671 | 11 (1.6) | 15 (2.2) |
Percentages appear in parentheses.
Irradiated and kept in confluency for 1 day.
Irradiated and kept in confluency for 14 days.
Irradiated and grown for 7 days.
Irradiated and grown for 14 days.
Results
γ-H2AX Foci Formation Monitors DSBs and Their Repair.
We investigated the induction and repair of x-ray-induced DSBs in primary human fibroblasts (MRC-5) in the G1 phase of the cell cycle by examining γ-H2AX foci formation and by PFGE. After IR doses of 2 or 0.2 Gy, small foci are visible as early as 3 min after irradiation and become more distinct after longer incubation times (Fig. 1A). Distributions of cells with a given number of foci are shown in Fig. 1C; the mean values after 3 min were 71 foci per cell for 2 Gy and 7.2 foci per cell for 0.2 Gy (Fig. 1E), for an average of 36 foci per Gy. PFGE studies performed in parallel in the dose range between 10 and 80 Gy yielded an initial number of 39 DSBs per Gy per cell, an estimate in remarkable agreement with the number of foci formed after 2 and 0.2 Gy and strong evidence for a one-to-one correlation between the number of γ-H2AX foci and IR-induced DSBs. This result suggests that other types of DNA damage induced by IR do not significantly contribute to γ-H2AX foci formation. It is important to note that the width of the distributions does not reflect uncertainties in foci counting but is indicative of the stochastic nature of focus induction by IR. This result is best seen by the fit of Poisson distributions to the 0.2-Gy data in Fig. 1 C and D. To gain further evidence that γ-H2AX foci formation can be used to monitor the presence of DSBs, we also analyzed the formation and disappearance of foci in confluent cultures of a DSB repair-deficient primary human fibroblast cell line, 180BR (17), which carries a defect in DNA ligase IV (18), an essential component of the major DSB repair machinery (Fig. 1B). The distribution of cells with foci after 3 min (Fig. 1D), the mean values of 70 foci per cell for 2 Gy and 7.7 foci per cell for 0.2 Gy (Fig. 1E), and the initial number of 38 DSBs per Gy per cell determined in PFGE studies all demonstrate that DSB induction and γ-H2AX foci formation are unaltered in 180BR cells compared with MRC-5 cells.
Significantly, although the initial number of foci was nearly identical, the number of foci present 24 h after irradiation with 2 or 0.2 Gy was clearly different between MRC-5 and 180BR cells. In MRC-5 cells, only a small fraction of the initial foci persists for 24 h whereas 180BR cells contain a substantial fraction of foci after this repair period (Fig. 1). Analysis of the time course for the disappearance of foci in MRC-5 and 180BR cells after exposure to 2 and 0.2 Gy showed that 180BR foci are lost with slower kinetics, compared with that observed in MRC-5 cells (Fig. 1E). PFGE studies performed in parallel demonstrate that the repair of DSBs after 10 and 80 Gy is profoundly defective in 180BR cells (Fig. 1F), consistent with previous measurements (15–17). Most importantly, for MRC-5 as well as for 180BR cells, the kinetics of foci disappearance closely resemble the kinetics of DSB repair. This finding provides further evidence that γ-H2AX foci represent DSBs and also shows that the dephosphorylation of γ-H2AX at the site of a DSB coincides temporally with the physical sealing of the break.
DSBs (Foci) Can Be Quantified After Doses as Low as 1 mGy.
We next determined the sensitivity of the γ-H2AX assay with regard to the lowest IR dose that is necessary to reliably quantify DSBs. As for all assays, the background level of damage present in the unirradiated sample determines the sensitivity of the γ-H2AX approach. We measured the background level of DSBs in confluent MRC-5 cells by counting γ-H2AX foci and obtained a value of ≈0.05 DSBs per cell, i.e., 1 in 20 cells contains a focus even without irradiation. Based on a DSB induction yield of 35–39 foci per cell per Gy, this value corresponds to a dose equivalent of 1.2–1.5 mGy. We therefore measured the induction of γ-H2AX foci in MRC-5 cells for a range of doses from 2 Gy to 1.2 mGy and obtained a linear relationship between the number of foci induced per cell and the IR dose delivered (Fig. 2). Also included in Fig. 2 are DSB induction yields for doses between 10 and 80 Gy obtained with PFGE measurements, the most sensitive approach available to quantify DSBs introduced by IR.
DSB Repair After Very Low Doses Is Substantially Compromised.
Previous studies with primary human fibroblasts using PFGE have demonstrated that the time course for repair of DSBs is independent of the initial x-ray dose (14). These studies were carried out at doses >10 Gy, and no information is available on cells incurring only a small number of DSBs. We investigated the kinetics of foci disappearance in confluent MRC-5 cells after doses of 20, 5, and 1.2 mGy and observed a decreasing capacity for DSB repair with decreasing IR dose (Fig. 3A). Whereas the time course of DSB repair after 20 mGy is similar to that observed after 200 mGy or 2 Gy, γ-H2AX foci induced by 5 mGy persist considerably longer. After exposure to 1.2 mGy, the number of foci per cell (Fig. 3A) as well as the distribution of cells with a given number of foci (Fig. 3B) does not change for repair times up to 24 h. This finding indicates a total lack of repair after 1.2 mGy.
Because the background level of γ-H2AX foci present in unirradiated controls is not negligible compared with the effects observed at very low IR doses, we determined the interexperimental variation in the level of spontaneous γ-H2AX foci for several cell lines and different culture conditions. Three primary human fibroblast cell lines were analyzed at early or late passage numbers either immediately or several weeks after the cells had reached confluency. In 15 independent determinations, the γ-H2AX background level was always between 0.04 and 0.06 foci per cell. In contrast, cells exposed to 20, 5, or 1.2 mGy and incubated for 24 h yield a level of persistent γ-H2AX foci between 0.09 and 0.11, which numerically corresponds to the initial number of foci present after a 1.2-mGy dose and is clearly different from the background level (Fig. 3C). This finding suggests that repair proceeds until a level of persistent DSBs of ≈0.1 foci per cell is reached. After a dose that corresponds to this persistent level (1.2 mGy), a lack of DSB repair is observed. If cells are exposed to doses that induce fewer initial DSBs than this persistent level (e.g., 0.5 or 0.1 mGy), <0.1 foci per cell are observed 24 h after irradiation (Fig. 3C). This finding demonstrates that the level of persistent foci is causally linked to initially induced DSBs and does not represent an artifactual cellular response to IR that is unrelated to DSBs. This conclusion is further substantiated by a cell cycle analysis using BrdUrd labeling, which shows that the γ-H2AX foci level of unirradiated und irradiated cultures is unrelated to S-phase cells (an unirradiated sample contained 43 foci in 787 cells, of which 10 cells were BrdUrd-positive and showed no foci; a 1.2-mGy + 24-h sample contained 54 foci in 521 cells with 10 BrdUrd-positive cells of which one contained one focus; a 20-mGy + 24-h sample contained 60 foci in 538 cells with 9 BrdUrd-positive cells that showed no foci).
We next investigated whether the level of ≈0.1 foci per cell can persist for incubation times >24 h. Cells that were exposed to 200, 20, 5, or 1.2 mGy and kept in confluency for the entire repair period show a level of ≈0.1 foci per cell for up to 4 days and even exhibit substantially more foci than the control for repair times up to 14 days (Fig. 4A). This finding shows that persistent DSBs can remain for many days and raises the question whether breaks induced by repeated irradiation on top of the level of persistent DSBs accumulate or whether cells can efficiently repair DSBs induced in excess of the ≈0.1-foci level. To address this point, confluent cells were irradiated over 10 days with daily doses of 1.2 or 5 mGy and analyzed for γ-H2AX foci 24 h after the last dose. Results from two different cell lines clearly show that DSBs induced by repeated irradiation do not accumulate above the level of ≈0.1 foci per cell (Fig. 4B). This finding further supports the idea that DSBs are efficiently repaired if they are in excess of this level, whereas repair at or below this level is strongly compromised.
Biological Consequences of Persistent DSBs.
Although the level of ≈0.1 foci per cell persists for several days, the data in Fig. 4A indicate a tendency of a slow foci loss for repair times of 7 and 14 days, particularly for the 5- and 1.2-mGy samples. We therefore investigated γ-H2AX foci in cells that were exposed in the confluent state to 5 or 1.2 mGy, but were trypsinized and reseeded at a 1:4 ratio at day 1 after irradiation, and a second time at day 7 when they had again reached confluency (Fig. 4C). Compared with confluent samples analyzed at day 1, cells allowed to grow after day 1 and analyzed at day 7 in the confluent state and also cells allowed to grow after day 1 and a second time after day 7, and then analyzed at day 14 in the confluent state show a γ-H2AX foci level similar to controls. This finding shows that the slow foci loss observed in confluent cultures is considerably enhanced for cells allowed to grow after irradiation.
It is possible that the slow loss of foci after many days of repair incubation does not represent the operation of a slow repair mechanism but indicates an elimination of the cells that carry a persistent DSB. This finding is supported by our observation (Table 1) that a 1.2- or 5-mGy dose increases the fraction of micronucleated and apoptotic cells in cultures allowed to grow for 7 or 14 days after irradiation (Fig. 4C) but not in cultures kept in confluency (Fig. 4A). We therefore tested cell survival by the colony-forming assay in confluent cultures that were irradiated with 1.2 mGy, 200 mGy, or 4 Gy and plated for survival 4 days after irradiation. Results from three independent experiments show a substantial cell-killing effect for all three doses (Table 2). We suggest that the significant reduction in cell survival after 1.2 mGy and at least part of the killing effect of the 200-mGy dose represent a direct biological consequence of the persistent DSBs.
Table 2.
Survival of MRC-5 cells
| Dose, mGy | Cell survival, %*
|
||
|---|---|---|---|
| Exp. I | Exp. II | Exp. III | |
| 0 | 100 ± 1 | 100 ± 2 | 100 ± 3 |
| 1.2 | 89 ± 4 | 92 ± 8 | 92 ± 5 |
| 200 | 77 ± 6 | 81 ± 1 | 75 ± 2 |
| 4,000 | 28 ± 1 | 23 ± 2 | 21 ± 2 |
Average ± SEM derived from triplicate samples of the optimal dilution.
Discussion
The observed quantitative similarity of induction of DSBs measured by PFGE and of γ-H2AX foci analyzed by immunofluorescence detection provides very strong evidence that a γ-H2AX focus represents a DSB. Additionally, for repair-proficient (MRC-5) as well as for repair-deficient (180BR) cells, the kinetics of foci disappearance closely resemble the kinetics of DSB repair. Thus, we conclude that immunofluorescence detection of γ-H2AX not only allows individual (and all) DSBs induced by IR to be visualized but also provides a quantitative measurement of the repair of individual breaks in single cells. This result was unexpected because murine DNA-dependent protein kinase catalytic subunit-knockout cells, which are also defective in DSB repair, show a pattern of γ-H2AX dephosphorylation with repair time similar to repair-proficient cells (6). This analysis, however, was carried out by Western blotting and may not provide the same sensitivity as measurement of foci formation.
We have analyzed the induction of γ-H2AX foci in MRC-5 cells between 1.2 mGy and 2 Gy and obtained a linear relationship between the number of foci per cell and the IR dose delivered. This finding provides direct evidence of a linear relationship between DSB induction and dose over this enormous dose range and supports other indirect evidence of a linear relationship that was obtained by using biophysical and biochemical approaches (19). It should be noted that doses in the order of 1–10 mGy are typically delivered with diagnostic x-ray exposures and represent the range of doses received by individuals per year due to environmental background radiation. At a dose of 1 mGy, a human fibroblast nucleus is traversed, on average, by one electron track so that further lowering the dose will not decrease the actual amount of damage received per single cell but will merely lower the fraction of cells hit by radiation particles (1).
Whereas the initial number of foci linearly depends on dose, the number of foci present after repair incubation does not. Instead, we observed that doses of 1.2, 5, 20, and 200 mGy all yield the same level of ≈0.1 persistent foci per cell (i.e., 1 focus per 10 cells) 24 h after irradiation. This level was obtained in cell lines with different passage numbers kept in confluency for various times before irradiation and was significantly different from the background value of ≈0.05 foci per cell (i.e., 1 focus per 20 cells). The level of ≈0.1 foci per cell corresponds to the initial number of foci observed in cells exposed to 1.2 mGy, and the excess above background of ≈0.05 foci per cell is, based on the linear relationship between DSB induction and dose demonstrated in Fig. 2, identical to the number of DSBs introduced by this dose.
The finding of a dose-independent level of persistent DSBs could theoretically be explained by a subpopulation of cells that develop foci during repair incubation either due to an apoptotic process (20) or by a mechanism known as “bystander” effect, in which signals transmitted from irradiated cells generate DNA damage in cells not directly hit (21–24). If this was the case, then the results presented would not indicate the presence of persistent DSBs but rather would be indicative of the generation of secondary breaks during repair incubation. Then, an increase in the number of DSBs over the number of breaks initially present might be expected after exposure to low doses. However, the mean number of DSBs per cell (Fig. 3A), as well as the distribution of cells with a given number of foci (Fig. 3B), remains constant with time after irradiation with 1.2 mGy, showing that secondary breaks have not arisen. Additionally, the number of DSBs present 24 h after irradiation with either 0.1 or 0.5 mGy does not exceed the number of breaks initially induced by these doses (Fig. 3C). We conclude from these observations that the persistent level of foci represents unrepaired DSBs. Because all cells that exhibit a DSB after exposure to 1.2 mGy show a lack of repair, the persistent level of unrepaired DSBs cannot be explained by a subpopulation of cells with compromised repair capacity.
We have further observed that the level of ≈0.1 foci per cell persists for several days if the cells are kept in confluency and that DSBs induced on top of this level by repeated irradiation are efficiently repaired. Thus, our data suggest the presence of a threshold level of damage above which repair mechanisms operate efficiently but at and below which cells are unable, or strongly impaired, to repair DSBs. Interestingly, this threshold level corresponds to a dose (≈1 mGy) at which a human fibroblast nucleus is traversed, on average, by approximately one electron track.
Currently, risk estimates for low doses of IR are based on empirical linear fits of existing human data determined at high doses. This extrapolation model assumes that cells have the capacity to repair IR damage at low doses as they do at high doses. Clearly, the data presented here do not support this assumption and could suggest that a linear extrapolation model significantly underestimates the risk for IR-induced carcinogenesis. However, it is important to consider that the presence of unrepaired DSBs was observed in nondividing confluent cell cultures. In an attempt to evaluate potential cellular consequences of residual DSBs, we irradiated cells in the confluent state and then allowed them to proliferate. After few cell divisions, irradiated cell cultures show nearly the same level of γ-H2AX foci but substantially more micronucleated and apoptotic cells than unirradiated controls, suggesting that cells with unrepaired DSBs are eliminated from the culture. This finding is supported by a significant reduction in cell survival after a dose of 1.2 mGy, an observation that may link the presence of residual DSBs to the phenomenon of low-dose hypersensitivity (25). From this point of view, it is tempting to speculate that the observed lack of DSB repair does not increase the carcinogenic risk of very low IR doses but rather represents a protective biological mechanism to reduce it. Instead of repairing a DSB in a particular cell with the risk of causing genetic alterations, it could be beneficial for an organism to remove the damaged cell and replace it by the division of an undamaged neighboring cell. Such a concept would be applicable to situations where a small fraction of the cells carry a DSB, so that repair is necessary only at higher damage levels. However, without an evaluation of the mutagenic potential of very low IR doses, this idea must remain speculative.
The observation that cells exposed to very low doses fail to repair the DSBs induced provides the opportunity to distinguish exposed from unexposed cell populations. The γ-H2AX assay as described here, therefore, has the potential to serve as a direct biomarker for human exposure to low quantities of IR. In addition to various applications in cancer therapy, the assay can be used to monitor exposure to occupational and diagnostic IR doses. With further automation, large cohorts can easily be examined. Its applicability, however, is not limited to the detection of IR-induced DSBs but includes other carcinogens that introduce DSBs.
Acknowledgments
We thank M. Kühne for performing PFGE measurements; R. Schepp for technical assistance; G. Kaul for introduction to immunofluorescence measurements; P. Jeggo for providing 180BR cells; K. Dittmann for providing HSF1 and HSF2 cells, and J. Kiefer, M. Montenarh, and P. Jeggo for critical comments on the manuscript. Part of this work was supported by the Bundesministerium für Bildung und Forschung via the Forschungszentrum Karlsruhe (Grant 02S8132).
Abbreviations
- DSB
double-strand break
- IR
ionizing radiation
- PFGE
pulsed-field gel electrophoresis
Footnotes
See commentary on page 4973.
References
- 1.United Nations Scientific Committee on the Effects of Atomic Radiation. UNSCEAR Sources and Effects of Ionizing Radiation: Report to the General Assembly, with Scientific Annexes. New York: United Nations Scientific Committee on the Effects of Atomic Radiation; 2000. [Google Scholar]
- 2.Hoeijmakers J H J. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 3.van Gent D C, Hoeijmakers J H J, Kanaar R. Nat Rev Genet. 2001;2:196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- 4.Rogakou E P, Pilch D R, Orr A H, Ivanova V S, Bonner W M. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 5.Rogakou E P, Boon C, Redon C, Bonner W M. J Cell Biol. 1999;146:905–915. doi: 10.1083/jcb.146.5.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burma S, Chen B P, Murphy M, Kurimasa A, Chen D. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 7.Chen H T, Bhandoola A, Difilippantonio M J, Zhu J, Brown M J, Tai X, Rogakou E P, Brotz T M, Bonner W M, Ried T, Nussenzweig A. Science. 2000;290:1962–1964. doi: 10.1126/science.290.5498.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen S, Casellas R, Reina-San-Martin B, Chen H T, Difilippantonio M J, Wilson P C, Hanitsch L, Celeste A, Muramatsu M, Pilch D R, et al. Nature. 2001;414:660–665. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahadevaiah S K, Turner J M, Baudat F, Rogakou E P, de Boer P, Blanco-Rodriguez J, Jasin M, Keeney S, Bonner W M, Burgoyne P S. Nat Genet. 2001;27:271–276. doi: 10.1038/85830. [DOI] [PubMed] [Google Scholar]
- 10.Modesti M, Kanaar R. Curr Biol. 2001;11:R229–R332. doi: 10.1016/s0960-9822(01)00112-9. [DOI] [PubMed] [Google Scholar]
- 11.Redon C, Pilch D, Rogakou E P, Sedelnikova O, Newrock K, Bonner W M. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 12.Sedelnikova O A, Rogakou E P, Panyutin I G, Bonner W M. Radiat Res. 2002;158:486–492. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Paull T T, Rogakou E P, Yamazaki V, Kirchgessner C U, Gellert M, Bonner W M. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 14.Löbrich M, Rydberg B, Cooper P K. Proc Natl Acad Sci USA. 1995;92:12050–12054. doi: 10.1073/pnas.92.26.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothkamm K, Kühne M, Jeggo P A, Löbrich M. Cancer Res. 2001;61:3886–3893. [PubMed] [Google Scholar]
- 16.Rief N, Löbrich M. J Biol Chem. 2002;277:20572–20582. doi: 10.1074/jbc.M200265200. [DOI] [PubMed] [Google Scholar]
- 17.Badie C, Goodhardt M, Waugh A, Doyen N, Foray N, Calsou P, Singleton B, Gell D, Salles B, Jeggo P A, et al. Cancer Res. 1997;57:4600–4607. [PubMed] [Google Scholar]
- 18.Riballo E, Critchlow S E, Teo S H, Doherty A J, Priestley A, Broughton B, Kysela B, Beamish H, Plowman N, Arlett C F, et al. Curr Biol. 1999;9:699–702. doi: 10.1016/s0960-9822(99)80311-x. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland B M, Bennett P V, Sidorkina O, Laval J. Proc Natl Acad Sci USA. 2000;97:103–108. doi: 10.1073/pnas.97.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogakou E P, Nieves-Neira W, Boon C, Pommier Y, Bonner W M. J Biol Chem. 2000;275:9390–9395. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 21.Prise K M, Belyakov O V, Folkard M, Michael B D. Int J Radiat Biol. 1998;74:793–798. doi: 10.1080/095530098141087. [DOI] [PubMed] [Google Scholar]
- 22.Nagasawa H, Little J B. Cancer Res. 1992;52:6394–6396. [PubMed] [Google Scholar]
- 23.Nagasawa H, Little J B. Radiat Res. 1999;152:552–557. [PubMed] [Google Scholar]
- 24.Zhou H, Randers-Pehrson G, Waldren C A, Vannais D, Hall E J, Hei T K. Proc Natl Acad Sci USA. 2000;97:2099–2104. doi: 10.1073/pnas.030420797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joiner M C, Marples B, Lambin P, Short S C, Turesson I. Int J Radiat Oncol Biol Phys. 2001;49:379–389. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]