Abstract
We use the oxygen isotopic composition of tooth enamel from multiple mammalian taxa across eastern Africa to present a proxy for aridity. Here we report tooth enamel δ18O values of 14 species from 18 locations and classify them according to their isotopic sensitivity to environmental aridity. The species are placed into two groups, evaporation sensitive (ES) and evaporation insensitive (EI). Tooth enamel δ18O values of ES animals increase with aridity, whereas the tooth enamel δ18O values of EI animals track local meteoric water δ18O values, demonstrating that bioapatite δ18O values of animals with different behaviors and physiologies record different aspects of the same environment. The enrichment between tooth enamel δ18O values of ES and EI animals records the degree of 18O enrichment between evaporated water (ingested water or body water) and source water, which increases with environmental aridity. Recognition of the ES–EI distinction creates the opportunity to use the 18O composition of bioapatite as an index of terrestrial aridity.
Keywords: bioapatite, East Africa, oxygen-18, mammals, water use
Terrestrial responses to major climate changes, such as glaciations, orogenic events, and shifts in ocean circulation, are often characterized in terms of water availability or aridity (1–3). Although aridity proxies exist for different terrestrial settings (4–6), they are not applicable in every circumstance and additional proxies must be developed for further study of terrestrial environmental change. The 18O composition of bioapatite has been used as a proxy for rainfall δ18O and seasonality of past environments (7, 8), but its utility in paleoenvironmental problems is limited by the complexity of climatic, environmental, physiological, and behavioral variables that influence bioapatite δ18O values. The correlation between bioapatite δ18O values and both meteoric water δ18O values and relative humidity demonstrate that animals have different isotopic responses to environmental change (7, 9, 10). In this study, we present tooth enamel δ18O data of 14 mammal species sampled from 18 locations in eastern Africa, which represent a gradient in environmental aridity. This data set shows that the tooth enamel δ18O values of some species vary with aridity whereas those of other species track the 18O composition of meteoric water. We use the different isotopic responses of the sampled species as the empirical basis for an aridity proxy.
Results
There is a marked increase in water deficit (WD) from the closed canopy Ituri Forest in the Democratic Republic of Congo to the arid shrublands of Lake Turkana in northern Kenya (Table 1, which is published as supporting information on the PNAS web site). WD in these regions negatively correlates to mean annual relative humidity (RH) (P < 0.01) and is used as a measure of aridity. Although previous studies compare bioapatite δ18O values with RH (6, 9, 10), RH data are not used here because they are not available for all study locations. Meteoric water δ18O (δ18Omw) values at these localities average −3.1‰ (SE, 1.2‰) and do not vary significantly with WD (P = 0.476). We use this average δ18Omw value for localities where water isotope data are not available.
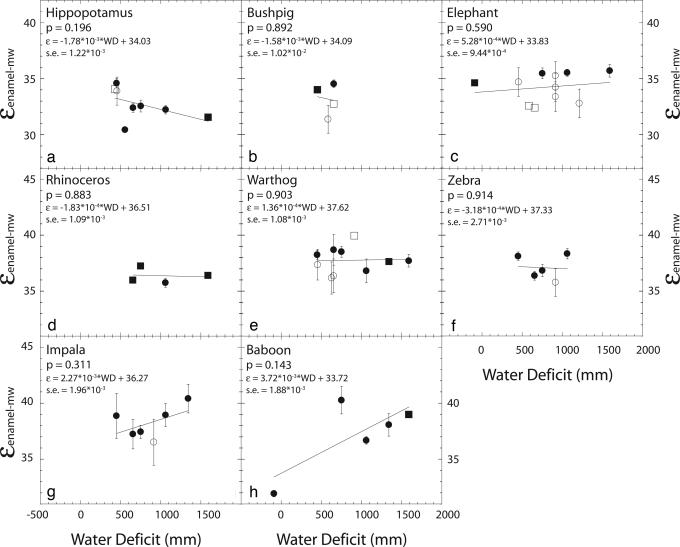
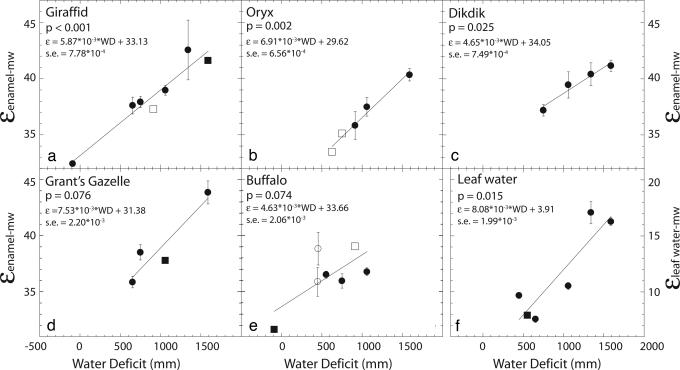
The isotopic enrichment between tooth enamel and meteoric water (εenamel-mw), where εA-B = ((RA/RB) − 1) × 1,000 is plotted as a function of WD (Figs. 1 and 2). The use of an enrichment factor, ε, enables tooth enamel δ18O (δ18Oenamel) values to be compared between sites, regardless of changes in δ18Omw values. εenamel-mw for hippopotamus (Hippopotamus amphibius), bush pig (Potomochoerus larvatus), elephant (Loxodonta africana), rhinoceros (Diceros bicornis), warthog (Phacochoerus africanus), zebra (Equus burchelli), impala (Aepyceros melampus), and baboon (Papio anubis), is not sensitive to changes in WD (P values range from 0.14 to 0.91) (Fig. 1). Baboons from Mpala are the only mixed (C3–C4) feeding baboons (otherwise C3 browsers), and when not considered the baboon regression has a P value <0.001 instead of 0.14. εenamel-mw of a second group, giraffids (Giraffa camelopardalis and Okapia johnstoni), oryx (Oryx beisa), dikdik (Madoqua kirkii), Grant’s gazelle (Gazella granti), and buffalo (Syncerus caffer), increases with WD (P values range from <0.001 to 0.08) (Fig. 2 a–e). The regression coefficients (slopes) of the second group do not vary significantly from each other (P > 0.1, F test).
Fig. 1.
εenamel-mw values for hippopotamus, bushpig, elephant, rhinoceros, warthog, zebra, impala, and baboon, plotted against water deficit (WD) (a–h). Circles represent εenamel-mw values calculated from average δ18Oenamel values at a location, whereas squares represent εenamel-mw values calculated from sites where the δ18Oenamel value from only one tooth was available. Error bars report the standard error associated with each ε value. No error bars are reported for εenamel-mw represented by squares. Filled symbols (●, ■) represent εenamel-mw values calculated from measured δ18Omw values, whereas open symbols (○, □) represent εenamel-mw values calculated from a regional δ18Omw average, −3.1‰. The linear regression, P value, and the standard error (s.e.) on the regression coefficient are reported for each plot.
Fig. 2.
εenamel-mw values (a–e) for giraffids, oryx, dikdik, Grant’s gazelle, and buffalo, and εlw-mw values (f), plotted against water deficit. εlw-mw values are only plotted for sites where δ18Omw and RH data are available. Symbols are the same as Fig. 1. The linear regression, P value, and the standard error (s.e.) on the regression coefficient are reported for each plot.
Discussion
The Evaporation Sensitive (ES)–Evaporation Insensitive (EI) Distinction.
The regressions between WD and εenamel-mw show that aridity affects δ18Oenamel values of some animals and not others. We assign the ES classification to those animals (giraffids, dikdik, and oryx) for which εenamel-mw is sensitive to WD (P < 0.05). εenamel-mw values of EI animals (hippopotamus, warthog, elephant, rhinoceros, and zebra) do not vary with aridity (P > 0.10). Although high regression coefficients (>10−3) suggest that the response of εenamel-mw values to WD for baboon, impala, Grant’s gazelle, and buffalo, is similar to that for ES taxa, these animals are placed in neither category because the regressions are not significant at the 0.05 level. ANOVA comparisons using a post hoc Scheffé test show that, within a single location, δ18Oenamel values of EI animals (hippopotamus, warthog, elephant/rhinoceros, and zebra) form significantly different groups (P < 0.05), whereas δ18Oenamel values of the ES animals (giraffids, dikdik, and oryx) do not vary significantly from each other (P > 0.05). Bushpig δ18Oenamel values are not included in the ES–EI classification because the data represent a very small range in WD.
The evaporative enrichment of 18O in ingested water and in body water has a strong influence on δ18O and δD values of both mammalian and avian body tissues, and it is a likely explanation for the sensitivity of ES εenamel-mw values to aridity (9–12). The 18O composition of leaf, surface, and body waters increase with greater evaporation, but the specific isotopic response of waters varies with boundary layer thickness, geometry of the evaporating water body, and the 18O composition of atmospheric water vapor (13). Of these waters, leaf water is most sensitive to evaporative enrichment in 18O (14, 15) and its relationship with WD represents a theoretical maximum slope between εenamel-mw and WD. To demonstrate the isotopic effect of evaporation on residual waters, we calculated leaf water δ18O (δ18Olw) values and εlw-mw by using a modified version of Craig and Gordon’s 1965 model (16). εlw-mw increases with WD (P = 0.02) (Fig. 2f).
We do not have the behavioral and physiological data that are necessary to calculate the flux of 18O/16O through the sampled animals and the relative effects of drinking, leaf and body waters on δ18Oenamel values of ES and EI animals, but we can use known behaviors and physiologies of these species to evaluate the contributions of these different waters to the δ18Oenamel values of the animals included in this study. The sensitivity of ES εenamel-mw values to aridity cannot be explained by evaporated drinking waters because (i) it is unlikely that ES animals consistently drink more water from evaporated sources than EI animals, and (ii) samples from animals known to drink evaporated waters were excluded from the data set (see Materials and Methods). Physiology and the evaporative enrichment of 18O in body water may have an important role in determining the sensitivity of δ18Oenamel values to aridity, but the diversity of ES animals, which are both large and small, panters and sweaters, and grazers and browsers (17–19), eliminates a single physiological explanation for the ES grouping.
The ingestion of evaporated leaf water is a viable explanation for increased εenamel-mw values of ES animals in water limited environments. ES animals identified in this study, oryx, giraffids, and dikdik, can survive with little or no drinking water and often obtain a substantial proportion of their water from leaves (17, 18, 20). In contrast, EI animals are associated with water, drink water daily, or consume nonleafy (nonevaporated) and moist portions of plants when water is less available (21–24). All of these behaviors would reduce the intake of evaporated relative to unevaporated waters. The proximity to water sources, large body size, skin that adsorbs water and mud, and wallowing behaviors of many EI animals may provide additional explanations for the isotopic buffering of EI animals from increased aridity (23, 25).
A combination of variables that affect the 18O evaporative enrichment of both leaf and body water is likely responsible for the ES and EI groupings. Fortunately, a specific mechanism for the groupings is not needed to develop δ18Oenamel values as indicators of aridity. The consistent slope for the ES εenamel-mw–WD regressions reflects a general relationship between evaporated waters and aridity that can be used to estimate aridity from δ18Oenamel values.
Aridity Index.
δ18Oenamel values of ES taxa track evaporative enrichment with respect to source water, whereas δ18Oenamel values of EI taxa track the 18O composition of source water. The enrichment between δ18Oenamel values from ES and EI animals (εES–EI) represents the degree of 18O enrichment between leaf or body water and meteoric water (εlw-mw or εbw-mw), which increases with aridity. Integrating two isotopic records to develop a proxy for aridity that controls for the isotopic composition of meteoric water is not a new concept; Cormie et al. (6) estimated RH by comparing bone collagen δD and δ18O values of bone collagen. Here, we modify the concept for application to δ18Oenamel values.
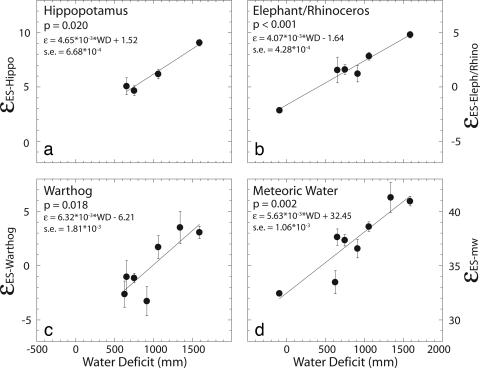
When δ18Oenamel values of ES animals are pooled, the isotopic enrichment between ES and EI δ18Oenamel values (εES–EI) varies significantly with WD for most EI animals such as hippopotamus, elephant/rhinoceros (pooled), and warthogs (P values <0.02) (Figs. 3a–c). The linear regression between εES–EI and WD is insignificant (P value = 0.2) for zebra, which may be explained by few εES–EI data points that lie within a small WD range. Among the significant WD-εES–EI regressions, the slopes do not significantly differ from each other (P > 0.1, F test), nor do they differ significantly from the slopes of regressions between WD and εES-mw or εlw-mw (P > 0.1, F test). We propose that the pooled or common slope of the WD-εES–EI regressions, 5.01E-03 (SE, 1.98E-03), is an aridity index that can be used to compare water deficit values within modern or fossil ecosystems. Application of the aridity index to the fossil record requires attention to the following three issues: identification of ES and EI taxa, sample size, and geologic context.
Identification of ES and EI species in the fossil record should be guided by behavioral information, morphology, carbon isotopic data, water dependency, and the ES–EI status of animals in modern analog systems. ES or EI status can be confirmed by using δ18Oenamel values themselves: εES–EI changes between sites that differ in water deficit, whereas εEI–EI and εES–ES do not.
Minimum sample size required for the aridity index is determined by a power analysis [significance (α) = 0.05, power level (1 − β) = 0.2, two-tailed test], wherein 10 samples are needed to determine a difference of 1.5‰ between populations of δ18Oenamel values with a standard deviation of 1.3‰, which is the average standard deviation of δ18Oenamel values for samples size >10. For the aridity index, this means that 10 ES and 10 EI specimens at one site are needed to determine water deficit values within 140 mm (using an aridity index slope of 5.01E-03 and determining the error for εES–EI by propagating the standard errors on mean δ18Oenamel values). Fewer samples are needed if less precision is acceptable. Clementz and Koch (26) calculated a similar sample size necessary to determine differences of 1‰ between mean δ18Oenamel values of terrestrial animals, also using a power analysis.
Establishing proper geological context is critical for application of the aridity index. εES–EI must be calculated from fossil teeth found within well constrained time intervals, preferably within one sedimentological stratum or well correlated unit. To reduce the possibility that animals accessed evaporated surface waters, fossils from lacustrine sediments should be avoided unless it can be demonstrated that the lake water was not enriched in 18O relative to meteoric water.
Fig. 3.
The isotopic enrichment (ε) between ES δ18Oenamel values (averaged values of all ES specimens at each site) and δ18Oenamel values of hippopotamus (a), elephant/rhinoceros (b), warthog (c), and meteoric water (d) is plotted with water deficit. ES and EI taxa are grouped based on ANOVA results presented in text. Linear regressions, P values, and the standard error (s.e.) on the regression coefficient are reported for each plot. Error bars represent the standard error associated with each ε value.
With the above criteria met, the aridity index can be used to convert differences in εES–EI between sites to differences in WD. The use of εES–EI and not absolute δ18Oenamel values eliminates the need to know taxon-specific fractionation factors between environmental water and body water δ18O values, and it controls for changes in δ18Omw values that are independent of aridity.
Conclusion
The empirical relationships presented here lay the foundation for a simple approach that relates δ18Oenamel values to aridity when it is not practical to model the flux of 18O/16O through an animal. The aridity index is a general relationship between aridity and δ18Oenamel values that is built on the distinction between ES and EI animals, wherein ES δ18Oenamel values track evaporation of leaf or body water and EI δ18Oenamel values track the 18O composition of meteoric water (drinking, stem, and root water). The isotopic enrichment between δ18Oenamel values of ES and EI animals, εES–EI, increases linearly with water deficit along a consistent slope, the aridity index, which can be used to calculate relative differences in water deficit through time or across space. The development of the aridity index with data from modern eastern African mammals makes the aridity index most immediately applicable to East African paleoenvironments, where aridity is considered to have influenced human evolution (27). However, the ES–EI distinction is a broadly applicable concept that can be used to evaluate animal water-use strategies in terrestrial systems where the 18O composition of bioapatite is unaltered.
Materials and Methods
In the past 10 years, modern mammal teeth from animals living within the past 45 years have been sampled from museum collections and field locations across eastern Africa for stable isotopic analysis. For this study, a subset of the larger tooth enamel isotopic data set (493 teeth of 1,000) was used to place better control on water sources accessed by animals (Table 2, which is published as supporting information on the PNAS web site). Tooth enamel δ18O (δ18Oenamel) values were only included in this subset if they were from animals with 18Oenamel results from four or more locations. Of these animals, 18Oenamel data were excluded if (i) they were from locations with large isotopic gradients (e.g., mountainous regions) or (ii) they were from animals known to drink evaporated water enriched in 18O with respect to local meteoric water.
Waters used to approximate δ18Omw values were sampled from sources with little evaporation (e.g., rivers with small catchments, springs, and shallow wells). Environmental aridity is characterized in terms of WD, the difference between potential evapotranspiration (PET) and mean annual precipitation (MAP), (WD = PET − MAP) using available data from references (28–31) and from N. Georgiadis (personal communication). PET was calculated from mean annual temperature and latitude (32).
Enamel was separated from dentine and powdered with a diamond or carbide bit following the long-axis of the tooth. Where multiple samples from each tooth were taken, the average value was used and considered as one sample. Enamel powders were pretreated by standard procedures in preparation for stable isotope analysis of the carbonate component of tooth enamel (33, 34). Waters were prepared for isotopic analysis by equilibration with CO2. Isotope ratios were measured on a Finnigan MAT 252 mass spectrometer at Stable Isotope Ratio Facility for Environmental Research, University of Utah. All isotopic ratios are reported in δ-notation, where δ = ((Rsample/Rstandard) − 1) × 1,000 and R is 18O/16O. δ values of tooth enamel are reported in reference to Vienna PeeDee Belemnite (VPDB), and waters are reported in reference to Vienna standard mean ocean water (VSMOW). For calculations in which it was necessary to convert values in the VPDB scale to the VSMOW scale, the following relationship was used: δ18OVSMOW = 1.3086 × δ18OVPDB + 30.86 (35). The enrichment between related isotopic values is reported as ε, where εA-B = ((RA/RB) − 1) × 1,000.
For sites where data were available, δ18Olw values were calculated by using a modified version of the 1965 Craig and Gordon evaporative enrichment model (13, 16, 36). To calculate δ18Olw values, mean annual temperature, mean annual RH (0600 h), and water δ18O values were used, and it was assumed that atmospheric humidity 18O composition is 9‰ lower than measured δ18Owater values, there is no temperature difference between the leaf and air, stomatal conductance = 0.3 m−2·s−1, and boundary layer conductance = 1 m−2·s−1. Statistics were computed with the statistical program JMP 4 (SAS Institute) and SYSTAT 10 (SPSS), or using methods described in Sokal and Rohlf (37).
Supplementary Material
Acknowledgments
We thank A. K. Behrensmeyer, F. H. Brown, C. S. Feibel, N. Georgiadis, J. A. Hart, L. N. Leakey, M. G. Leakey, R. F. Leakey, N. Mudida, R. Potts, J. G. Wynn, the Kenya Wildlife Service, and the National Museums of Kenya for assistance in collecting specimens. We thank P. Koch, M. Kohn, and H. Fricke for reviews of the manuscript. This research was conducted with support from the Packard Foundation.
Abbreviations
- WD
water deficit
- RH
relative humidity
- ES
evaporation sensitive
- EI
evaporation insensitive.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Sirocko F., Seelos K., Schaber K., Rein B., Dreher F., Diehl M., Lehne R., Jager K., Krbetschek M., Degering D. Nature. 2005;436:833–836. doi: 10.1038/nature03905. [DOI] [PubMed] [Google Scholar]
- 2.Dettman D. L., Fang X. M., Garzione C. N., Li J. J. Earth Planet. Sci. Lett. 2003;214:267–277. [Google Scholar]
- 3.Cane M. A., Molnar P. Nature. 2001;411:157–162. doi: 10.1038/35075500. [DOI] [PubMed] [Google Scholar]
- 4.Wilf P. Geol. Soc. Am. Bull. 2000;112:292–307. [Google Scholar]
- 5.Retallack G. J. Geology. 2005;33:333–336. [Google Scholar]
- 6.Cormie A. B., Luz B., Schwarcz H. P. Geochim. Cosmochim. Acta. 1994;58:3439–3449. [Google Scholar]
- 7.Longinelli A. Geochim. Cosmochim. Acta. 1984;48:385–390. [Google Scholar]
- 8.Fricke H. C., Clyde W. C., O’Neil J. R. Geochim. Cosmochim. Acta. 1998;62:1839–1850. [Google Scholar]
- 9.Luz B., Cormie A. B., Schwarcz H. P. Geochim. Cosmochim. Acta. 1990;54:1723–1728. [Google Scholar]
- 10.Ayliffe L. K., Chivas A. R. Geochim. Cosmochim. Acta. 1990;54:2603–2609. [Google Scholar]
- 11.McKechnie A. E., Wolf B. O., del Rio C. M. Oecologia. 2004;140:191–200. doi: 10.1007/s00442-004-1564-9. [DOI] [PubMed] [Google Scholar]
- 12.Hobson K. A., Atwell L., Wassenaar L. I. Proc. Natl. Acad. Sci. USA. 1999;96:8003–8006. doi: 10.1073/pnas.96.14.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig H., Gordon L. I. Stable Isotopes in Oceanographic Studies and Paleotemperatures. Pisa: V. Lischi; 1965. pp. 9–130. [Google Scholar]
- 14.Gonfiantini R., Gratziu S., Tongiorgi E. Use of Isotopes and Radiation in Soil-Plant Nutrition Studies. Vienna: International Atomic Energy Agency; 1965. pp. 405–409. [Google Scholar]
- 15.Sternberg L. d. S. L. In: Stable Isotopes in Ecological Research. Rundel P. W., Ehleringer J. R., Nagy K. A., editors. New York: Springer; 1989. pp. 124–141. [Google Scholar]
- 16.Roden J. S., Ehleringer J. R. Plant Physiol. 1999;120:1165–1173. doi: 10.1104/pp.120.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maloiy G. M. O. Proc. R. Soc. London B; 1973. pp. 167–178. [DOI] [PubMed] [Google Scholar]
- 18.Pellew R. A. J. Zool. Soc. London. 1984;202:57–81. [Google Scholar]
- 19.Taylor C. R. Sci. Am. 1969;220:89–95. doi: 10.1038/scientificamerican0169-88. [DOI] [PubMed] [Google Scholar]
- 20.Taylor C. R. Nature. 1968;219:181–182. doi: 10.1038/219181a0. [DOI] [PubMed] [Google Scholar]
- 21.Sinclair A. R. E. The African Buffalo. Chicago: The University of Chicago Press; 1977. [Google Scholar]
- 22.Western D. E. Afr. Wildlife J. 1975;13:265–286. [Google Scholar]
- 23.Rodgers W. A. Mammalia. 1984;48:327–350. [Google Scholar]
- 24.Schenkel R., Schenkel-Hulliger L. Ecology and Behavior of the Black Rhinoceros (Diceros bicornis L.) Hamburg: Verlag Paul Parey; 1969. [Google Scholar]
- 25.Lillywhite H. B., Stein B. R. J. Zool. (London) 1987;211:727–734. [Google Scholar]
- 26.Clementz M. T., Koch P. L. Oecologia. 2001;129:461–472. doi: 10.1007/s004420100745. [DOI] [PubMed] [Google Scholar]
- 27.deMenocal P. B. Earth Planet. Sci. Lett. 2004;220:3–24. [Google Scholar]
- 28.East African Meteorological Department. Climatological Statistics for East Africa. Nairobi, Kenya: East African Meteorological Department; 1975. [Google Scholar]
- 29.East R. Afr. J. Ecol. 1984;22:245–270. [Google Scholar]
- 30.Altmann J., Alberts S. C., Altmann S. A., Roy S. B. Afr. J. Ecol. 2002;40:248–251. [Google Scholar]
- 31.Cerling T. E., Hart J. A., Hart T. B. Oecologia. 2004;138:5–12. doi: 10.1007/s00442-003-1375-4. [DOI] [PubMed] [Google Scholar]
- 32.Thornthwaite C. W. Geogr. Rev. 1948;38:55–94. [Google Scholar]
- 33.Lee Thorp J., van der Merwe N. J. S. Afr. J. Sci. 1987;83:712–715. [Google Scholar]
- 34.Koch P. L., Tuross N., Fogel M. L. J. Arch. Sci. 1997;24:417–429. [Google Scholar]
- 35.Friedman I., O’Neil J. R. Compilation of Stable Isotope Fractionation Factors of Geochemical Interest. Reston, VA: U.S. Geological Survey; 1977. pp. 1–12. [Google Scholar]
- 36.Cappa C. D., Hendricks M. B., DePaolo D. J., Cohen R. C. J. Geophys. Res. Atmos. 2003;108 doi:10.1029/2003JD003597. [Google Scholar]
- 37.Sokal R. R., Rohlf F. J. Biometry. New York: Freeman; 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.