Abstract
Calcium ions are important to some prokaryotic cellular processes, such as heterocyst differentiation of cyanobacteria. Intracellular free Ca2+concentration, [Ca2+]i, increases several fold in heterocysts and is regulated by CcbP, a Ca2+-binding protein found in heterocyst-forming cyanobacteria. We demonstrate here that CcbP is degraded by HetR, a serine-type protease that controls heterocyst differentiation. The degradation depends on Ca2+ and appears to be specific because HetR did not digest other tested proteins. CcbP was found to bind two Ca2+ per molecule with KD values of 200 nM and 12.8 μM. Degradation of CcbP releases bound Ca2+ that contributes significantly to the increase of [Ca2+]i during the process of heterocyst differentiation in Anabaena sp. strain PCC 7120. We suggest that degradation of CcbP is a mechanism of positive autoregulation of HetR. The down-regulation of ccbP in differentiating cells and mature heterocysts, which also is critical to the regulation of [Ca2+]i, depends on NtcA. Coexpression of ntcA and a ccbP promoter-controlled gfp in Escherichia coli diminished production of GFP, and the decrease is enhanced by α-ketoglutarate. It was also found that NtcA could bind a fragment of the ccbP promoter containing an NtcA-binding sequence in a α-ketoglutarate-dependent fashion. Therefore, [Ca2+]i is regulated by a collaboration of HetR and NtcA in heterocyst differentiation in Anabaena sp. strain PCC 7120.
Keywords: cyanobacteria, protease
Cyanobacteria appeared on Earth ≈2.5–3 billion years ago (1). The release of oxygen as a by-product of photosynthetic electron transfer by the cyanobacteria led to a fundamental change of the biosphere. The accumulation of oxygen in the environment greatly stressed many organisms because oxygen is highly toxic to many biochemical reactions that could only be carried out under anaerobic conditions. Nitrogenase, for example, is an enzyme that is sensitive to oxygen, and biological nitrogen fixation could only take place in the absence of oxygen molecules. One of the mechanisms by which cyanobacterial nitrogen fixation adapted to an oxidizing environment was the restriction of nitrogenase to specialized cells called heterocysts (2–6). Heterocysts have several means for protection of nitrogenase from oxygen molecules: a thick envelope to limit oxygen penetration, the absence of photosystem II so that no oxygen is evolved, and a high respiratory rate to consume oxygen. In some cyanobacteria, heterocysts are distributed in a semiregular pattern along the filaments.
One of the signals that triggers the differentiation from a vegetative cell to a heterocyst in response to nitrogen deprivation is the increase of the intracellular concentration of α-ketoglutarate (2-OG) (7, 8). Another important signal in heterocyst differentiation is the intracellular concentration of free calcium, [Ca2+]i (9, 10). It is known that Ca2+ ions play very important roles in cellular processes in eukaryotes and that eukaryotic [Ca2+]i is tightly regulated and maintained in the nanomolar range. Although the role of Ca2+ in prokaryotic cellular activities is less clear (11–13), current evidence also shows that [Ca2+]i is also tightly regulated in bacteria (14, 15) and that Ca2+ plays important roles in bacterial cell differentiation such as sporulation of Bacillus (16) and heterocyst formation of cyanobacteria (10). It was recently shown that [Ca2+]i increases in differentiating cells after transfer from a nitrogen-replete condition to a nitrogen-deprived condition (10). CcbP, a calcium-binding protein in heterocyst-forming cyanobacteria, plays an important role in the regulation of [Ca2+]i and is absent in mature heterocysts. The expression of ccbP is also down-regulated in heterocysts (10). However, it is not known at present how Ca2+ is released from CcbP and how the expression of ccbP is regulated.
The initiation of heterocyst differentiation is controlled by key genes hetR (17) and ntcA (18, 19). hetR encodes a serine-type protease with DNA-binding activity (20, 21). Even though it has been shown that HetR is autodegrading, no other substrates of HetR have been found so far. One important feature of hetR is that its expression is positively autoregulatory (22). Although the binding of the hetR promoter by HetR dimer could be important to the autoregulatory process (21), the mechanism of the autoregulation is not well understood. NtcA is a transcription factor that belongs to the cAMP receptor protein superfamily and positively regulates the expression of many genes involved in cell differentiation (23). Its DNA-binding activity is regulated by 2-OG (24). NtcA also has been shown to negatively regulate rbcL encoding the large subunit of Rubisco (25). Recent evidence has shown that the expression of ntcA and hetR is mutually dependent (26).
In this report, we show that CcbP from Anabaena sp. strain PCC 7120 (hereafter referred to as Anabaena 7120) is specifically degraded by HetR and that the degradation depends on Ca2+. We also demonstrate that NtcA is involved in the down-regulation of ccbP in a 2-OG-dependent fashion.
Results
Degradation of CcbP by HetR and the Release of Bound Calcium Ions.
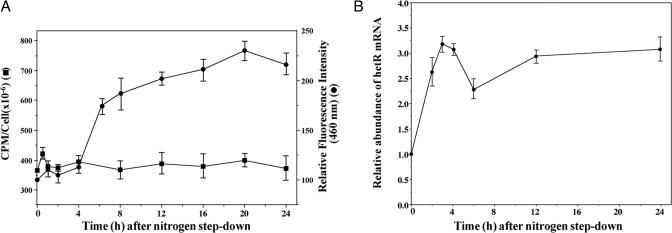
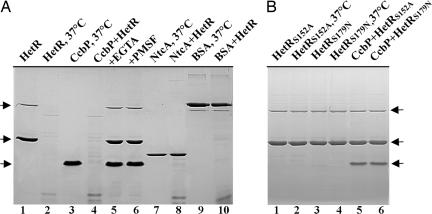
It has been demonstrated that mature heterocysts and proheterocysts have an increased [Ca2+]i (10). To understand the mechanism for regulation of [Ca2+]i during heterocyst differentiation, we studied the kinetics of [Ca2+]i increase during heterocyst differentiation. Fig. 1A shows that a small increase of [Ca2+]i could be observed within 1 h after nitrogen step-down. However, the major increase of [Ca2+]i occurred 4 h after nitrogen deprivation. Fig. 1A also shows that, whereas [Ca2+]i increased 2-fold in Anabaena 7120 during the process of heterocyst differentiation as shown by the intensity of obelin-catalyzed coelenteramide fluorescence, which depends on Ca2+ (27), the overall cellular Ca2+content remained unchanged during this period based on assays with radioactive 45Ca2+. These results indicated that the increase of [Ca2+]i during the heterocyst differentiation of Anabaena 7120 was not a result of an up-regulated Ca2+ uptake but likely a consequence of the release of bound Ca2+. Because CcbP is a major Ca2+-binding protein in Anabaena 7120, we determined the cellular concentration of CcbP by ELISA, and the CcbP concentration was 0.036 (±0.008) fg per cell, or 2.5 μM. Because CcbP is absent in heterocysts and heterocysts have a relatively high [Ca2+]i, the results shown in Fig. 1 suggest that the increase of [Ca2+]i in differentiating cells and mature heterocyst could be a result of CcbP degradation. The time course of the increase of [Ca2+]i is ≈1–2 h behind that of hetR induction, which reached its maximum level 3 h after nitrogen deprivation (Fig. 1B). Because HetR is a protease, we tested whether CcbP could be a substrate of HetR. The results are shown in Fig. 2. Incubation of HetR at 37°C led to an autodigestion as previously demonstrated (20). Incubation of CcbP with HetR at 37°C resulted in the complete digestion of CcbP and HetR. Because CcbP was not digested if HetR was absent (Fig. 2A, lane 3), the degradation of CcbP (Fig. 2A, lane 4) was not due to digestion by contaminating proteases. The degradation of both proteins could be prevented by 5 mM EGTA (Fig. 2A, lane 5) and by 0.2 mM PMSF (Fig. 2A, lane 6), a serine-type protease inhibitor. The degradation of CcbP by HetR was probably specific because NtcA of Anabaena 7120 and BSA were not degraded by HetR (Fig. 2A, lanes 7–10). HetRS152A and HetRS179N are two mutant HetR proteins that show no autodegradation, and the strains carrying these mutant genes could not initiate heterocyst differentiation (17, 28). Incubation of CcbP with HetRS152A or HetRS179N did not result in any digestion of CcbP, demonstrating that the active serine of HetR is required for the degradation of CcbP (Fig. 2B). HetRC48A, a mutant protein that cannot form dimer but retains protease activity, can digest CcbP (data not shown).
Fig. 1.
Measurement of Ca2+ content, cellular free Ca2+ concentration, and expression of hetR during heterocyst differentiation of Anabaena 7120. (A) Measurement of cellular Ca2+ content. Total cellular Ca2+ of Anabaena 7120 (■) was determined with 45Ca2+, and [Ca2+]i was determined with Ca2+-dependent fluorescence emission at 460 nm in Anabaena 7120 expressing the obelin gene (●). The values of fluorescence emission were normalized to the initial value at time 0, i.e., when combined nitrogen was removed. (B) Relative amount of the hetR mRNA during heterocyst differentiation as determined by quantitative PCR. Each point represents an average of six individual measurements, and all values were normalized to the value at time 0 of nitrogen step-down.
Fig. 2.
SDS/PAGE analysis of CcbP degradation by HetR. (A) Degradation of CcbP by wild-type HetR under the conditions indicated above the gel. The duration of incubation at 37°C was 2 h. The initial concentrations of HetR, CcbP, NtcA, and BSA were 1, 2, 1, and 2 mg·ml−1, respectively. The concentrations of EGTA and PMSF were 5 and 0.2 mM, respectively. Lanes: 1 and 2, HetR before and after incubation at 37°C, respectively; 3 and 4, CcbP after incubation without or with HetR at 37°C, respectively; 5 and 6, CcbP after incubation with HetR in the presence of 5 mM EGTA or 0.2 mM PMSF, respectively; 7 and 8, NtcA after incubation without or with HetR at 37°C, respectively; 9 and 10, BSA after incubation without or with HetR at 37°C, respectively. The thin bands below the major BSA bands in lanes 9 and 10 were from BSA stock and were present before treatment. (B) No digestion of CcbP by two mutant HetR proteins that lack autodegradation activity. The concentrations of CcbP, HetRS152A, and HetRS179N and the digestion conditions were the same as for CcbP and HetR in A. Lanes: 1 and 2, HetRS152A before and after incubation at 37°C, respectively; 3 and 4, HetRS179N before and after incubation at 37°C, respectively; 5 and 6, incubation of CcbP with HetRS152A and HetRS179N at 37°C, respectively. The upper arrows in A and B indicate the position of the HetR dimer, the middle arrows indicate the position of HetR monomer, and the lower arrows indicate the position of CcbP.
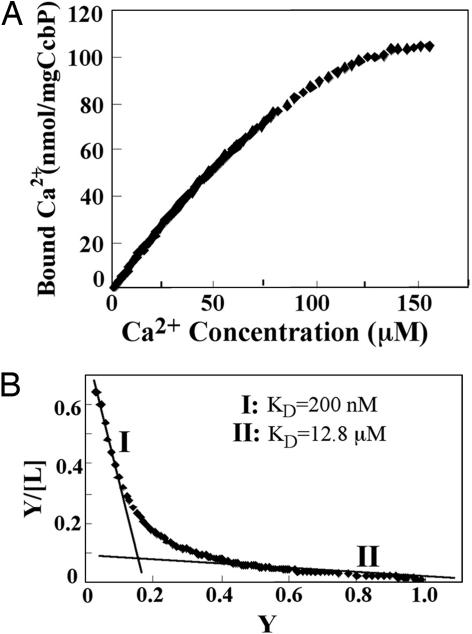
The release of CcbP-bound Ca2+ during the process of CcbP degradation by HetR was investigated (Fig. 3). The initial concentration of CcbP was adjusted to 3 μM, similar to the CcbP concentration in vivo. Once HetR was added, free Ca2+ concentration increased rapidly from 1 μM to 7 μM, although no such increase was observed when HetR was omitted (Fig. 3). If the initial free Ca2+ concentration was adjusted to 1 μM in the absence of CcbP, addition of HetR to the solution did not lead to an increase of free Ca2+ concentration. This result indicated that complete degradation of 1 μmol of CcbP could release ≈2 μmol of Ca2+. Measurement of the Ca2+/CcbP ratio showed that one CcbP binds approximately two (1.73 ± 0.41) Ca2+ (Fig. 4A). The Kd values for CcbP’s two Ca2+-binding sites were 12.8 μM and 200 nM (Fig. 4B). It is estimated that the intracellular concentration of the CcbP-bound Ca2+ is ≈1.5 μM based on the fact that the cellular concentration of CcbP was 2.5 μM and the fact that the physiological concentrations of free Ca2+ are between 100 nM and 200 nM (15). These results demonstrate that the Ca2+ released from CcbP by HetR digestion could contribute significantly to the increase of [Ca2+]i during heterocyst differentiation.
Fig. 3.
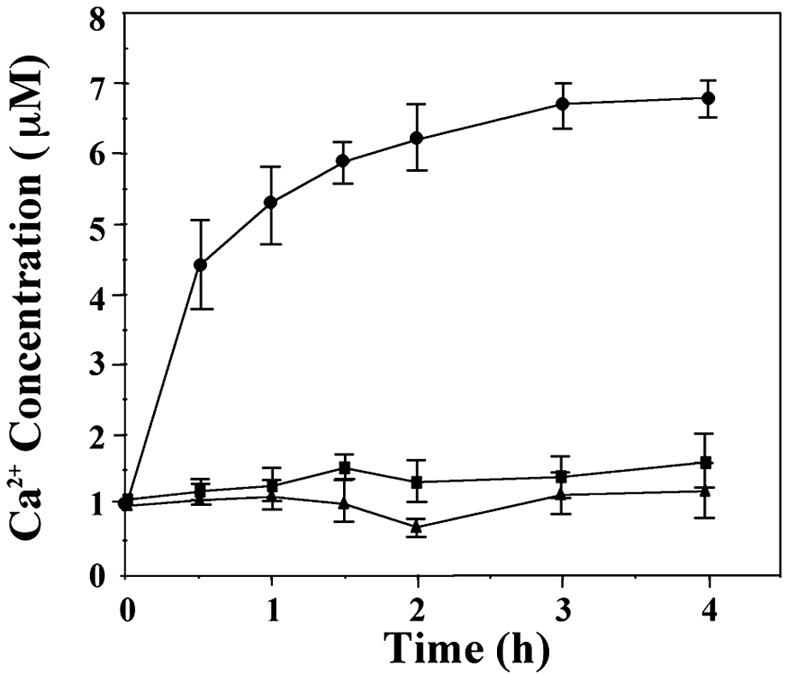
Release of bound Ca2+ from CcbP during its digestion by HetR. Solutions (50 mM Tris·HCl, pH 7.4/100 mM KCl) containing both HetR at 0.1 mg·ml−1 and CcbP at 0.43 mg·ml−1 (3 μM) (●), HetR only (■), or CcbP only (▴) were incubated at 37°C, and the concentrations of free Ca2+ were measured with a Ca2+ electrode. The initial concentration of free Ca2+ was adjusted to 1.0 μM.
Fig. 4.
Measurement of the number of bound Ca2+ per molecule of CcbP. (A) Determination of the stoichiometry of CcbP and bound Ca2+. CaCl2 from a stock solution of 1 M was added incrementally to a 10-ml 0.83 mg·ml−1 CcbP solution in 10 mM Tris·HCl buffer (pH 7.5) containing 100 mM KCl, and free Ca2+ in solution was measured with a Ca2+ electrode. An average of 1.7 Ca2+ bound per CcbP was determined. (B) Scatchard plot for the determination of Ca2+ dissociation constants (Kd) of CcbP. Y represents the percentage of CcbP with bound Ca2+ and [L] represents the free Ca2+ concentration in micromolar. Curves I and II were obtained by curve fitting. Curve I has a slope of −4.99, corresponding to a Kd of 200 nM; curve II has a slope of −0.078, corresponding to a Kd of 12.8 μM.
Down-Regulation of the Expression of ccbP During Heterocyst Differentiation.
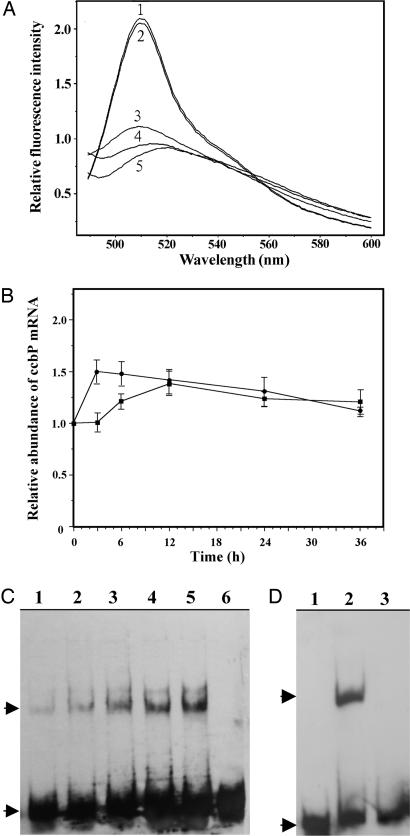
It has been shown that the expression of ccbP in heterocysts is down-regulated, which could be critical to the increase of [Ca2+]i in heterocysts and proheterocysts (10). Analysis of the ccbP promoter region of Anabaena 7120 showed that there was a potential NtcA-binding site, GTTCTGAGTGGTCACA (23), 154 bp upstream of the start codon of ccbP (nucleotides in bold indicate the conserved binding sequence). To investigate whether NtcA was directly involved in the regulation of ccbP expression, we studied the effect of coexpression of ntcA and gfp (encoding GFP) controlled by the ccbP promoter from Anabaena 7120 in Escherichia coli (Fig. 5). When E. coli cells containing the plasmid pPccbP-gfp, which bears a gfp gene under control of the ccbP promoter (10), were excited with a blue light (460 nm), a GFP-specific emission spectrum was obtained, suggesting that the ccbP promoter is functional in E. coli and that functional GFP was produced. When the E. coli cells containing both pPccbP-gfp and pET-ntcA were grown in the presence of 0.1 mM isopropyl β-d-thiogalactoside (IPTG) to induce the expression of ntcA, emission of GFP was reduced by ≈70%. If the medium contained 0.5 mM 2-OG as well as 0.1 mM IPTG, emission from GFP was not detected. When E. coli cells containing pPccbP-gfp and pET-psaE, which was used for production of PsaE of photosystem I (29), were induced with IPTG and 2-OG, emission of GFP was same as that from E. coli containing only pPccbP-gfp. These results indicate that NtcA negatively regulated the activity of the ccbP expression in E. coli and that this regulation was influenced by 2-OG. The role of NtcA on the ccbP expression of Anabaena 7120 was investigated by measuring the amount of ccbP mRNA after nitrogen step-down in both the wild-type and ntcA− strains (Fig. 5B). The ccbP mRNA in the wild type remained unchanged within the first 3 h after nitrogen step-down. In contrast, the ccbP transcript in ntcA− increased by 50% in the same period, indicating that NtcA negatively regulates ccbP expression in the early stage of heterocyst differentiation. The amount of ccbP mRNA in both strains reached to the same level 12 h after nitrogen step-down followed by a gradual decrease. Whether NtcA affected the activity of the ccbP promoter was further tested by EMSA using DNA fragments in the ccbP promoter region that contain the possible NtcA-binding sequence noted before. As shown in Fig. 5C, the presence of 3 nM recombinant NtcA in the binding buffer resulted in a retardation of migration of the DNA fragment in gel electrophoresis (Fig. 5C, lanes 1–5), whereas BSA alone did not have such an effect (Fig. 5C, lane 6). The presence of increasing concentrations of 2-OG (Fig. 5C, lanes 1–5) in the binding buffer led to an increasing amount of DNA retarded in electrophoresis, indicating that 2-OG enhanced interaction of NtcA with the DNA fragment. Fig. 5D shows that the NtcA-binding sequence (GTN11ACA) in the ccbP promoter region was required for NtcA-induced gel mobility shift of a synthetic DNA fragment (Fig. 5D, lane 2). When the binding sequence was absent, no retardation of the DNA band was observed (Fig. 5D, lane 3).
Fig. 5.
Down-regulation of the ccbP gene of Anabaena 7120 by NtcA. (A) GFP fluorescence spectra of E. coli cells expressing a gfp gene under control of the ccbP promoter and the ntcA gene from Anabaena 7120 inducible by IPTG. Curves: 1, fluorescence spectrum obtained from the E. coli cells containing pPccbP-gfp; 2, emission spectrum obtained when the E. coli cells containing pPccbP-gfp and pET-psaE were in the presence of 0.1 mM IPTG and 0.5 mM 2-OG; 3 and 4, emission spectra obtained when the E. coli cells containing both pPccbP-gfp and pET-ntcA were in the presence of 0.1 mM IPTG without or with 0.5 mM 2-OG, respectively; 5, a spectrum obtained from E. coli cells containing pRL25C (from which pPccbP-gfp is derived) and pET-ntcA. No GFP fluorescence emission peak was obtained. The optical densities at 600 nm of all cultures were adjusted to 1.0 before the measurement of the fluorescence spectra. (B) Quantitative PCR analysis of ccbP expression in the wild type (■) and ntcA− (●) after nitrogen step-down. Total RNA was isolated at the times indicated for quantitative PCR. All values were normalized to that at time 0. (C) NtcA-induced gel mobility shift of a 100-bp DNA fragment in the ccbP promoter region of Anabaena 7120. DNA (400 ng) was incubated with 3 nM NtcA in the binding buffer with 2-OG at the concentrations described below for 10 min before analysis with polyacrylamide gel (6%) electrophoresis. Lanes: 1–5, incubation of the DNA fragment with NtcA in the presence of 2-OG at concentrations of 0, 0.05, 0.1, 0.2, and 0.5 mM, respectively; 6, the DNA fragment incubated with BSA alone. (D) The NtcA-binding sequence was required for the NtcA-induced gel mobility shift. The conditions for EMSA were the same as in C, except that synthetic DNA fragments of the ccbP promoter region (from nucleotides −179 to −130 upstream of the start codon) were used. The sequence GTN11ACA was retained in one of the fragments (lane 2), and it was changed to CCN11CCC in the other fragment (lane 3). The conditions for lane 1 were the same as those for lane 2, except that no NtcA was included in the binding buffer. (C and D) The upper arrows indicate the positions of the shifted DNA bands and the lower arrows indicate the free DNA fragments.
Discussion
Free [Ca2+]i increases in differentiating cells and mature heterocysts, and the increase of free [Ca2+]i is required for the process of heterocyst differentiation (10). CcbP is a recently identified calcium-binding protein present in Anabaena 7120 and other heterocyst-forming cyanobacteria. CcbP regulates heterocyst differentiation by sequestering Ca2+. In this study, we focused our investigation on these two aspects of the regulation of [Ca2+]i by CcbP during heterocyst formation: A mechanism for the release of the CcbP-bound Ca2+ during the process of differentiation and a mechanism for down-regulation of the ccbP expression in heterocysts.
Although CcbP lacks apparent Ca2+-binding motifs, such as EF hands, it binds two Ca2+ per molecule (Fig. 4). Based on the cellular concentration of CcbP as determined by ELISA, the CcbP-bound Ca2+ is a significant pool of Ca2+, and they could increase [Ca2+]i 6- to 8-fold if completely released. The reduced amount of CcbP in heterocysts (10) suggests that CcbP is degraded during differentiation.
HetR, a serine-type protease (20), has been recognized as the master switch of heterocyst differentiation (17). Although HetR could be specifically labeled by the serine-type protease inhibitors and showed autodegradation (20), no other physiological substrate of its protease activity was known. In this report, we demonstrate that HetR can degrade CcbP (Fig. 2). Although the mechanism of degradation of CcbP by HetR is not entirely clear, the reaction appears specific because HetR digests neither BSA nor NtcA. Among many proteins, such as phycobiliproteins, PsaE, and PsaD, CcbP was the only protein digested by HetR. Both autodigestion of HetR and degradation of CcbP were dependent on Ca2+ because EGTA completely prevented both reactions. The active serine of HetR (Ser-152) was required to digest CcbP. This result is consistent with the conclusion that HetR is a serine-type protease and that the active serine is required for heterocyst differentiation (28). Fig. 2 also demonstrates that HetRS179N was unable to digest CcbP, indicating that, although Ser-179 is not the active serine, it is required for the protease activity. This suggestion is in agreement with early reports that no heterocysts were formed in the strain carrying hetRS179N (17) and that HetRS179N showed no autodegradation (20).
Together with PatS (30, 31), HetN (31–35), and PatA (36, 37), HetR controls heterocyst pattern. One of the critical factors for the control of pattern formation is positive autoregulation (38). Although it has been demonstrated that the expression of hetR is positively autoregulated (22), how the positive feedback of HetR is achieved was not clear. The evidence that HetR regulates [Ca2+]i (Fig. 3) provides a mechanism for achieving the positive autoregulation of HetR at posttranslational level because its enzymatic activity depends on Ca2+ (Fig. 2). It is likely that digestion of CcbP by HetR under physiological conditions is positively autoregulatory because the released Ca2+ would stimulate HetR activity when [Ca2+]i is low. The increased [Ca2+]i could play other important roles in heterocyst differentiation, such as Ca2+-dependent proteolysis (39). Heterocyst-forming cyanobacteria contain many regulatory proteins, such as kinases and enzymes for cyclic nucleotides, and Ca2+ may also regulate the activities of some of these enzymes.
Down-regulation of ccbP in heterocysts, which also led to an increase of [Ca2+]i, depended on NtcA (Fig. 5). Although only a minimal NtcA-binding sequence was present in the region of the ccbP promoter (23), NtcA bound to the fragment containing this sequence (Fig. 5). rbcL of Anabaena 7120 is negatively regulated by NtcA (25). The NtcA-binding sequence of ccbP, like that of rbcL, is located downstream of a putative −10 box of a predicted promoter (our unpublished results). Therefore, the down-regulations of ccbP and of rbcL by NtcA are similar. As in Synechococcus sp. PCC 7942 (24), the NtcA-binding activity was enhanced by 2-OG based on assays of the GFP reporter gene in vivo and gel mobility shifting in vitro (Fig. 5). These results suggest that 2-OG also is involved in the regulation of [Ca2+]i, enforcing its signaling in the initiation of heterocyst differentiation (8). The difference of ccbP expression after nitrogen deprivation between the wild type and ntcA− (Fig. 5) suggests that repression of ccbP expression in the initiation stage of heterocyst differentiation could be critical to the increase of [Ca2+]i. The expression of ntcA and hetR in heterocyst differentiation is mutually dependent, and the up-regulation of hetR requires NtcA (24, 26). Because the hetR promoter contains no NtcA-binding sequence, it is generally believed that the regulation of hetR expression by ntcA is indirect. The results shown in Fig. 5 suggest that the regulation of ccbP expression by NtcA could contribute to the regulation of hetR up-regulation by NtcA because it contributes to the increase of [Ca2+]i. The increase of [Ca2+]i in differentiating cells is likely due to the release of CcbP-bound Ca2+, although it cannot be ruled out that some Ca2+ could be imported from vegetative cells. The degradation of CcbP by HetR assures that only those differentiating cells with high HetR content would increase their [Ca2+]i, whereas [Ca2+]i in vegetative cells remains low. The results in Fig. 2 show that HetR significantly degrades itself in vitro. Because HetR is likely to be modified in vivo (37, 40, 41), autodegradation of HetR may be prevented in differentiating cells and heterocysts in vivo.
In this study, we demonstrate that HetR, CcbP, and NtcA collaborate in the control of [Ca2+]i in heterocyst differentiation. We predict that the identification of CcbP as a substrate of HetR will help with the understanding of the proteolytic mechanism of HetR. The digestion of CcbP by HetR for increasing [Ca2+]i may represent a primitive mechanism for the regulation of [Ca2+]i because it requires complete digestion of a Ca2+-binding protein, whereas more sophisticated mechanisms of Ca2+ homeostasis are evolved in eukaryotic cells (42).
Materials and Methods
Strains and Growth Conditions.
Anabaena 7120 was grown in BG11 or BG110 media illuminated with cool fluorescent light (43). E. coli was grown in LB medium at 37°C. The strain DH5α was used for all general cloning purposes. The strain BL21(DE3) was used for protein overproduction and for coexpression of ntcA of Anabaena 7120 and gfp.
DNA Manipulation and Protein Overproduction.
The sequences of primers used in this study and the procedures for overproduction of NtcA and HetR are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. CcbP was overproduced as described previously (10). The NtcA-induced DNA mobility shift was performed according to Huang et al. (21). A100-bp fragment from the ccbP promoter for EMSA was amplified by PCR with primers 1 and 2 (see Table 1, which is published as supporting information on the PNAS web site). The DNA bands after polyacrylamide gel (6%) electrophoresis were visualized by x-ray films (Kodak). To confirm whether the sequence GTN11ACA in the ccbP promoter region was required for NtcA binding, EMSA was performed with two synthetic DNA fragments based on the DNA sequence from nucleotides −179 to −130 upstream of the start codon of the ccbP gene. The GTN11ACA sequence was changed to CCN11CCC in one of the fragments. Coexpression of the ntcA of Anabaena 7120 and gfp was carried out by transformation of E. coli strain BL21(DE3) with pET-ntcA or pET-psaE (29), both of which confer resistance of ampicillin, and pPccbP-gfp, which contains the gfp gene under control of the ccbP promoter (10) and confers resistance to kanamycin, with selection on ampicillin and kanamycin. Quantitative PCR for determination of hetR and ccbP mRNA in Anabaena 7120 was performed according to Huang et al. (21). The primers used for determination of ccbP transcripts were primers 3 and 4 (Table 1). The values obtained with quantitative PCR were normalized to that obtained by quantitative PCR from 16S rRNA with primers 5 and 6 (Table 1).
Characterization of CcbP.
The stoichiometry of CcbP and its bound Ca2+ was determined as follows. To a 10-ml solution containing 0.83 mg·ml−1 CcbP, 10 mM Tris·HCl (pH 7.5), and 100 mM KCl, portions of a 1 M stock solution of CaCl2 were added incrementally, and free Ca2+ in solution was measured with a Ca2+ electrode according to Baudet et al. (44). The amount of Ca2+ per CcbP was determined based on the titration curve. To calculate the Ca2+ dissociation constants (Kd) of CcbP, Scatchard plotting of the above-described titration was determined and curves were fit with the software Sigmaplot (Systat). To measure the release of bound Ca2+ from CcbP during its digestion by HetR, solutions (50 mM Tris·HCl, pH7.4/100 mM KCl) containing HetR at 0.1 mg·ml−1 and CcbP at 0.43 mg·ml−1 (3 μM), HetR only, or CcbP only were incubated at 37°C, and the free Ca2+ concentrations were determined. The initial free Ca2+ concentration was adjusted to 1.0 μM.
Cellular concentration of CcbP was determined by ELISA with a Protein Detector Elisa kit from KPL (Gaithersburg, MD). Total soluble proteins of Anabaena 7120 were serially diluted in the coating buffer. The amount of CcbP was determined by using rabbit anti-CcbP antibodies as primary antibodies according to the instruction of the supplier. The cellular concentration of CcbP was estimated according to Laurent et al. (8) in their estimation of cellular concentrations of 2-OG.
Detection of [Ca2+]i.
Ca2+-dependent fluorescence emission by obelin was detected as described by Zhao et al. (10). Changes of fluorescence emission at 460 nm were used to determine changes of [Ca2+]i in Anabaena 7120. Estimation of the total cellular calcium content of Anabaena 7120 was performed by 45Ca2+ labeling according to Smith et al. (45).
Supplementary Material
Acknowledgments
We thank Dr. E. Flores (Instituto de Bioquimica, Seville, Spain) for providing the ntcA− strain. This study was supported by National Natural Science Foundation of China Grants 30230040 and 30540017 and Ministry of Science and Technology of China Grant 01CB108903.
Abbreviations
- IPTG
isopropyl-β-d-thiogalactopyranoside
- 2-OG
α-ketoglutarate.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Golubic S., Sergeev V. N., Knoll A. H. Lethaia. 1995;28:285–298. doi: 10.1111/j.1502-3931.1995.tb01817.x. [DOI] [PubMed] [Google Scholar]
- 2.Buikema W. J., Haselkorn R. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1993;44:33–52. [Google Scholar]
- 3.Wolk C. P. In: Prokaryotic Development. Brun Y. V., Shimkets L. J., editors. Washington, DC: Academic; 2000. pp. 83–104. [Google Scholar]
- 4.Meeks J. C., Elhai J. Microbiol. Mol. Biol. Rev. 2002;66:94–121. doi: 10.1128/MMBR.66.1.94-121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golden J. W., Yoon H. S. Curr. Opin. Microbiol. 2003;6:557–563. doi: 10.1016/j.mib.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C.-C., Laurent S., Sakr S., Peng L., Bédu S. Mol. Microbiol. 2006;59:367–375. doi: 10.1111/j.1365-2958.2005.04979.x. [DOI] [PubMed] [Google Scholar]
- 7.Li J. H., Laurent S., Konde V., Bedu S., Zhang C.-C. Microbiology. 2003;149:3257–3263. doi: 10.1099/mic.0.26462-0. [DOI] [PubMed] [Google Scholar]
- 8.Laurent S., Chen H., Bédu S., Ziarelli F., Peng L., Zhang C.-C. Proc. Natl. Acad. Sci. USA. 2005;102:9907–9912. doi: 10.1073/pnas.0502337102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torrecilla I., Leganés F., Bonilla I., Fernández-Piñas F. Microbiology. 2004;150:3731–3739. doi: 10.1099/mic.0.27403-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Y., Shi Y., Zhao W., Huang X., Wang D., Brown N., Brand J. J., Zhao J. Proc. Natl. Acad. Sci. USA. 2005;102:5744–5748. doi: 10.1073/pnas.0501782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youatt J. Crit. Rev. Microbiol. 1993;19:83–97. doi: 10.3109/10408419309113524. [DOI] [PubMed] [Google Scholar]
- 12.Michiels J., Xi C., Verhaert J., Vanderleyden J. Trends Microbiol. 2002;10:87–93. doi: 10.1016/s0966-842x(01)02284-3. [DOI] [PubMed] [Google Scholar]
- 13.Dominguez D. C. Mol. Microbiol. 2004;54:291–297. doi: 10.1111/j.1365-2958.2004.04276.x. [DOI] [PubMed] [Google Scholar]
- 14.Gangola P., Rosen B. P. J. Biol. Chem. 1987;262:12570–12574. [PubMed] [Google Scholar]
- 15.Torrecilla I., Leganés F., Bonilla I., Fernández-Piñas F. Plant Physiol. 2000;123:161–175. doi: 10.1104/pp.123.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbaud M. L., Guiseppi A., Denizot F., Haiech J., Kihoffer M. C. Biochim. Biophys. Acta. 1998;1448:212–226. doi: 10.1016/s0167-4889(98)00145-1. [DOI] [PubMed] [Google Scholar]
- 17.Buikema W. J., Haselkorn R. Genes Dev. 1991;5:321–330. doi: 10.1101/gad.5.2.321. [DOI] [PubMed] [Google Scholar]
- 18.Frias J. E., Flores E., Herrero A. Mol. Microbiol. 1994;14:823–832. doi: 10.1111/j.1365-2958.1994.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 19.Wei T.-F., Ramasubramanian T. S., Golden J. W. J. Bacteriol. 1994;176:4473–4482. doi: 10.1128/jb.176.15.4473-4482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou R., Wei X., Jiang N., Li H., Dong Y., Hsi K. L., Zhao J. Proc. Natl. Acad. Sci. USA. 1998;95:4959–4963. doi: 10.1073/pnas.95.9.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang X., Dong X., Zhao J. Proc. Natl. Acad. Sci. USA. 2004;101:4848–4853. doi: 10.1073/pnas.0400429101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black T. A., Cai Y., Wolk C. P. Mol. Microbiol. 1993;9:77–84. doi: 10.1111/j.1365-2958.1993.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 23.Herrero A., Muro-Pastor A. M., Valladares A., Flores E. FEMS Microbiol. Rev. 2004;28:469–487. doi: 10.1016/j.femsre.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Tanigawa R., Shirokane M., Maeda Si S., Omata T., Tanaka K., Takahashi H. Proc. Natl. Acad. Sci. USA. 2002;99:4251–4255. doi: 10.1073/pnas.072587199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramasubramanian T. S., Wei T. F., Golden J. W. J. Bacteriol. 1996;176:1214–1223. doi: 10.1128/jb.176.5.1214-1223.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muro-Pastor A. M., Valladares A., Flores E., Herrero A. Mol. Microbiol. 2002;44:1377–1385. doi: 10.1046/j.1365-2958.2002.02970.x. [DOI] [PubMed] [Google Scholar]
- 27.Markova S. V., Vysotski E. S., Blinks J. R., Burakova L. P., Wang B. C., Lee J. Biochemistry. 2002;41:2227–2236. doi: 10.1021/bi0117910. [DOI] [PubMed] [Google Scholar]
- 28.Dong Y., Huang X., Wu X. Y., Zhao J. J. Bacteriol. 2000;182:1575–1579. doi: 10.1128/jb.182.6.1575-1579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J., Snyder W. B., Muhlenhoff U., Rhiel E., Warren P. V., Golbeck J. H., Bryant D. A. Mol. Microbiol. 1993;9:183–194. doi: 10.1111/j.1365-2958.1993.tb01680.x. [DOI] [PubMed] [Google Scholar]
- 30.Yoon H. S., Golden J. W. Science. 1998;282:935–938. doi: 10.1126/science.282.5390.935. [DOI] [PubMed] [Google Scholar]
- 31.Callahan S. M., Buikema W. J. Mol. Microbiol. 2001;40:941–950. doi: 10.1046/j.1365-2958.2001.02437.x. [DOI] [PubMed] [Google Scholar]
- 32.Black T. A., Wolk C. P. J. Bacteriol. 1994;176:2282–2292. doi: 10.1128/jb.176.8.2282-2292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer C. C., Ramaswamy K. S., Endley S., Scappino L. A., Golden J. W., Haselkorn R. FEMS Microbiol. Lett. 1997;151:23–30. doi: 10.1016/s0378-1097(97)00128-6. [DOI] [PubMed] [Google Scholar]
- 34.Li B., Huang X., Zhao J. FEBS Lett. 2002;517:87–91. doi: 10.1016/s0014-5793(02)02582-6. [DOI] [PubMed] [Google Scholar]
- 35.Borthakur P. B., Orozco C. C., Young-Robbins S. S., Haselkorn R., Callahan S. M. Mol. Microbiol. 2005;57:111–123. doi: 10.1111/j.1365-2958.2005.04678.x. [DOI] [PubMed] [Google Scholar]
- 36.Liang J., Scappino L., Haselkorn R. Proc. Natl. Acad. Sci. USA. 1992;89:5655–5659. doi: 10.1073/pnas.89.12.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orozco C. C., Risser D. D., Callahan S. M. J. Bacteriol. 2006;188:1808–1816. doi: 10.1128/JB.188.5.1808-1816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meinhardt H., Gierer A. BioEssays. 2000;22:753–760. doi: 10.1002/1521-1878(200008)22:8<753::AID-BIES9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 39.Lockau W., Massalsky B., Dirmeier A. Eur. J. Biochem. 1988;172:433–438. doi: 10.1111/j.1432-1033.1988.tb13906.x. [DOI] [PubMed] [Google Scholar]
- 40.Buikema W. J., Haselkorn R. Proc. Natl. Acad. Sci. USA. 2001;98:2729–2734. doi: 10.1073/pnas.051624898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou R., Cao Z., Zhao J. Arch. Microbiol. 1998;169:417–423. doi: 10.1007/s002030050592. [DOI] [PubMed] [Google Scholar]
- 42.Clapham D. E. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 43.Rippka R., Deruelles J., Waterbury J. B., Herdman M., Stanier R. Y. J. Gen. Microbiol. 1979;111:1–61. [Google Scholar]
- 44.Baudet S., Hove-Madsen L., Bers D. M. Methods Cell Biol. 1994;40:93–113. [PubMed] [Google Scholar]
- 45.Smith R. J., Hobson S., Ellis I. R. New Phytol. 1987;105:531–541. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.